* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 10~chapter_8_electro..
Power engineering wikipedia , lookup
Cavity magnetron wikipedia , lookup
Grid energy storage wikipedia , lookup
Mercury-arc valve wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Multi-junction solar cell wikipedia , lookup
Electric battery wikipedia , lookup
Distributed generation wikipedia , lookup
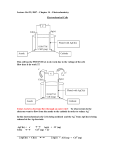
Electrochemical Engineering & Alternate Energy How to store some electricity for later use Layout of this lecture • Green energy – Load leveling • Electrochemical principles – Anodes and cathodes – Half cells and simple electrochemical cells • Fuels Cells • Ragone plot and battery capacities Why electrochemical engineering? • Batteries and fuel cells are deeply embedded in “Green Energy” because solar and wind energy systems need to store electrical energy • It’s also a hot topic because some vehicles use batteries for propulsion such as in hybrid cars and trucks • Electrochemical engineering is the study of what happens inside batteries, fuel cells and ‘ultracapacitors’ – So be prepared for a little more chemistry! Green Energy • “Green energy” refers to renewable energy supplies that do not spew forth greenhouse gases nor toxic impurities – Wind and solar energy are two favored sources • Big disadvantage #1: Only works when the wind blows or the sun shines • Big disadvantage #2: May make too much electricity exactly when you don’t need it. • Solution: Store the electrical energy until you do need it. Load leveling – These may be very large batteries! Power demand Evening power demand Time Power produced • Electricity not used when generated, nor available when needed if the sun or wind go down • Solution: Battery storage Morning power demand Noon peak generation Time Figure 2: Mismatch daily power production Mismatch of greenofelectric power and use and Solar or wind need a lot of equipment Batteries Why do batteries work? • Matter is inherently electrically charged. –Simplest case is ionic bonding (i.e., attraction) in compounds such as common table salt: Na+Cl- in which Coulombic forces hold together positively charged sodium ions and negatively charged chlorine ions. The force between these ions is: 2 e F 2 kr where e is the charge on an electron and r is the interionic distance. k is the dielectric constant, which is ~80 for water. Electrochemistry • When Na+Cl- dissolves in water with k ~ 80, the forces between ions lessen allowing free ions as Na+ and as Cl– Ion-containing solutions are called “Electrolytes” – Overall ion-containing solutions are electrically neutral – Locally ion-containing solutions have both charged species at short distances to each other – Batteries use these ions when they can be separated Electrolytes, Anodes and Cathodes • Electrodes are classified as anodes and cathodes • Anodes are “sources” of electrons, and cathodes are “sinks” for electrons – – – – e- = electrons E = Electrolyte C = Cathode A = Anode e- flow - + C A E Discharge through a load Electrolytes, Anodes and Cathodes • Anodes are “sources” of electrons, and cathodes are “sinks” for electrons – – – – e- = electrons E = Electrolyte C = Cathode A = Anode e- flow + C A E Charge through an source Electrolytes, Anodes and Cathodes • Beware Franklin’s error! – – – – e- = electrons E = Electrolyte C = Cathode A = Anode Conventional Current flow - + C A E Discharge through a load Lead-Acid Batteries • These are the batteries you find in a car – Both electrodes are based on lead, Pb one with a PbO2 coating – The electrolyte is sulfuric acid written H2SO4, which dissolved in water is 2H+ /SO=4 (the sulfate ion has two electrons/molecule) – The principle anodic reaction is: Pb Pb++ + 2e– The two electrons flow through the external circuit to the cathode on which: PbO2 4H SO 2e PbSO4 2H2 0 4 Lead-Acid Batteries • Product of reaction is PbSO4 which precipitates during discharge and dissolves during charging. • The anodic voltage at the anode is 0.36V above a reference cell and the cathodic is 1.69 V below. • Overall cell voltage = ~2.0 V C A Anodic 0.36 V Reference cell E Cathodic 1.69V Can you power a car using batteries alone? Property Mass Energy Mass energy storage density Volumetric energy storage density Power Lead-acid battery 25. kg 3,000 kJ 120 kJ/kg 250 kJ/liter Gasoline 25. kg 1.2 105 kJ 46,500 kJ/kg 34,400 kJ/liter 5 kW Typically >100 kW • This battery is too heavy, contains too little energy, and delivers too little power – that’s why hybrids are a popular substitute. The Electrochemical Series • Cell voltage set by the tendency to transfer electrons Half Cell Chemistry Li+ + e- Li(s) Na+ + e- Na(s) Mg++ + 2e- Mg(s) Zn++ + 2 e- Zn(s) Fe++ + 2 e- Fe(s) Ni++ + 2e- Ni(s) 2H+ + 2 e- H2(g) Cu++ + 2 e- Cu(s) Cu+ + e- Cu(s) Ag+ + e- Ag(s) Pd++ + 2e- Pd(s) Potential in volts –3.05 V –2.71 V –2.37 V –0.76 V –0.44 V –0.25 V 0.00V (Hydrogen ½ cell is defined as zero) 0.34 V 0.52 V 0.80 V 0.95 V The Electrochemical Series • These half-cell reactions in principle are reversible. The more negative the more they want to lose electrons; the more positive the more to gain them – This determines how cells will behave: Daniell cell • The electrolytes are ZnSO4(aq) with a Zn anode and CuSO4(aq) with a Cu cathode. Write down the reactions in each half cell and explain what happens in the salt bridge. What is the voltage? Daniell cell • In bulk aqueous solution , Zn++ and SO4= must be in balance with each other. ZnSO4 Aq Zn SO 4 • But the anode is also dissolving and thus yields some locally extra Zn++ ions according to: Zn(s) Zn 2e (V 0.76V) Daniell cell • Electrons from the anode flow through the external circuit precipitating copper at the cathode: Cu 2e Cu s 0.34V • We have removed copper ions from solution; therefore there must be a corresponding reduction in SO4= ions from the electrolyte. They must move into the salt bridge to exactly counteract the Zn++ ions from the anodic side. The cell potential is equal to: (+0.76 V) + (+0.34 V) = +1.10 V. Electroplating • An electroplater wants to coat a 10.0 cm by 10.0 cm copper plate with 12.5 micrometers of silver. How many electrons must pass in the external circuit? How many coulombs are passed? If the plating takes 1,200. s what’s the electrical current in amperes in the external circuit? Electroplating • Know: Atomic mass of Ag is 108 kg/kmol. Its density is 10,500 kg/m3. Avogadro’s number (NAv) is 6.02 1026 atoms/kmol. What we call current is nothing but the rate of flow of electrons, so 1.00 A = 1.00 coulomb/s and one electron carries –1.60 10-19 coulombs. Conventional current flow + - Electron flow Ag Cu Ag+, NO3- , H2O Electroplating • The reaction at the anode is Ag(s) → Ag+ + eand the reaction at the cathode is Ag+ + e- → Ag(s); hence one atom of silver dissolves at the anode and one atom of silver is deposited at the cathode. For each atom of silver dissolving at the anode and depositing at the cathode, one electron must circulate in the external circuit. Electroplating 2 12.5×10-6 ×100.×10,500 2 3 cm m Mass = μm cm kg/m / 2 μm m 1.00×104 = 1.31×10-3 kg • Next convert to kmols: kmols 1.31 10 3 5 kg kmols 1 . 22 10 kmols 108 kg Electroplating • Next convert to atoms of Ag(s): Ag atoms deposited 1.22 10 5 6.02 10 26 7.32 10 21[kg][ atoms / kg] 7.32 10 atoms 21 Electroplating • Next convert to mass of Ag(s): 7.32 10 21 1.6010 19[e ][coulombs/e - ] 1.17 10 coulombs 3 1.17 10 Hence current [C/s ] 0.975A 1200. 3 Fuel Cells • Fuel cells are just continuously refueled batteries. – They will not discharge while electrochemical fuel is being fed to them. • Most fuel cells depend on “Proton Exchange Membrane” or “PEM” to catalyze electrode reactions 2 H 2 4 H 4e Anodic Rxn 4 H 4e O2 2 H 2OH 2 H 2 0 Cathodic Rxn Fuel Cells http://aq48.dnraq.state.ia.us/prairie/images Fuels cells • Note: Only H2 and O2(i.e.,air) in and only H2O out. • Cell voltage is 1.23 V for overall rxn H2 + O2 = H2O – Apparently no green house gas pollution! – Unfortunately to make H2 needs copious CO2 The Ragone Chart • Batteries must supply both energy and power – Typically batteries supply current measured at mA/cm2 of electrode area at a few volts – The more electrode area, the greater the current; this may be internal area packing or simply more cells placed in parallel – The more cells in series the higher the voltage • But it’s the application that demands whether the cell can deliver both enough energy (say mileage between charges on an electric car) and power (say to pass another car) The Ragone Chart • The best measures of energy and power efficiency are their mass densities: e = E/Wt (Whr/kg) and p = P/Wt (W/kg) • The energy density delivered by a power source for a time t is simply e = p t. – Take log base 10 of this equation: • log10 e = log10 p + log10 t – Plot the log10 energy density of a battery vs. log10 power density for the same battery and you get the Ragone Plot The Ragone Chart Modified from a graphic of Maxwell Technologies: http://www.maxwell.com. The Ragone Chart • Very convenient way to compare different electrochemical sources – Ideally you want to have your cake and eat it by being in the upper right corner – Reality shows what can be achieved by competing electrochemical sources • “Ultracapacitors” are storage devices that can store thousands of times the energy capability of an electrical capacitor. Ragone Chart 1,000 rs u o 10h our h 1 100 Energy, Whr/kg • That that the form of log10 e = log10 p + log10 t is y = mx + c and has a slope of m =1 given the log10 scaling in chart. • The battery’s discharge time is given by e/p s 0 6 3 10 s 36 1 0.1 s 3.6 s m 6 6s 3 . 0 3 0.01 10 100 Power, W/kg 1,000 10,00 Summary: • Green energy and load storage and leveling • Electrochemical series – Simple electrical cells – Simple electrochemistry • Principles of fuel cells • Ragone chart to characterize





































![Cells_and_Batteries[1]](http://s1.studyres.com/store/data/008447161_1-940a99f6b74a9937747db18f0db2a1c2-150x150.png)