* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Critical Reviews in Oral Biology & Medicine
Survey
Document related concepts
Transcript
Critical Reviews http://cro.sagepub.com/
in Oral Biology & Medicine
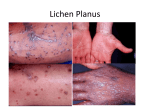
The Therapy of Oral Lichen Planus
Drore Eisen
CROBM 1993 4: 141
DOI: 10.1177/10454411930040020101
The online version of this article can be found at:
http://cro.sagepub.com/content/4/2/141
Published by:
http://www.sagepublications.com
On behalf of:
International and American Associations for Dental Research
Additional services and information for Critical Reviews in Oral Biology & Medicine can be found at:
Email Alerts: http://cro.sagepub.com/cgi/alerts
Subscriptions: http://cro.sagepub.com/subscriptions
Reprints: http://www.sagepub.com/journalsReprints.nav
Permissions: http://www.sagepub.com/journalsPermissions.nav
>> Version of Record - Jan 1, 1993
What is This?
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Critical Reviews in Oral Biology and Medicine, 4(2):141-158 (1993)
The Therapy of Oral Lichen Planus
Drore Eisen, D.D.S., M.D.
Dermatology Associates of Cincinnati, Inc., Mercy Health Plaza, Beechmont at Five Mile Road,
7691 Five Mile Road, Cincinnati, OH 45230
ABSTRACT: Oral lichen planus is a chronic mucocutaneous disease that is relatively common. Although many
patients are asymptomatic and require no therapy, those who exhibit atrophic and erosive lesions are often a
challenge to treat. All therapies are palliative, and none is effective universally. Currently employed treatment
modalities include corticosteroids administered topically, intralesionally, or systemically. Alternative therapies
include topical and systemic retinoids, griseofulvin, Cyclosporine, and surgery. Other medical treatments and
experimental modalities, including mouth PUVA, have been reported to be effective. Controversy concerning the
efficacy of all these treatments suggests that oral lichen planus is a heterogeneous disorder. Eliminating lichenoid
drug eruptions, candidiasis, trauma, contact mucositis, and emotional stress may play a role in the management
of these patients. This article is a review of the many treatments and measures that have been employed in the
management of patients with oral lichen planus.
KEY WORDS: oral lichen planus, corticosteroids, retinoids, Cyclosporine, griseofulvin, Dapsone, PUVA.
I. INTRODUCTION
Oral lichen planus is not a rare disease, as
evidenced by large series of patients reported from
various institutions. Although the true prevalence
is unknown, various studies suggest an incidence
of 1 to 2% of the general population.
Several generalizations can be made about
the clinical characteristics of this disease based
on observations in published reports (Table 1).
Oral lichen planus affects women twice as often
as men and occurs most commonly in the fifth to
sixth decades of life. Although many cases are
asymptomatic and discovered upon routine oral
examination, roughly two thirds of patients report oral discomfort upon presentation of their
disease.
Andreasen (1968) divided oral lichen planus
into six clinical forms, including reticular, papular, plaquelike, atrophic, erosive, and bullous. A
simpler classification system we have devised
involves grouping reticular, papular, and
plaquelike oral lichen planus into reticulated lesions, which usually are asymptomatic. Erosive
(including bullous) lesions and atrophic
(erythematous) lesions are distinct forms that are
usually a source of pain and discomfort.
Desquamative gingivitis, a clinically descriptive
term that encompasses many diseases, can apply
to conditions characterized by clinically atrophic
lesions with histologic oral lichen planus
(McCarthy et a/., 1960; Rogers et al, 1982).
Lichen planus may be found on any mucosal
surface including the esophagus and larynx, but
lesions have a predilection for the posterior buccal mucosa and tongue. The gingiva and lips are
also commonly affected. Concomitant lesions
usually are found, and lesions may change forms
during the course of the disease.
Unfortunately, oral lichen planus is a chronic
disease. Complete remissions are either nonexistent or infrequent, especially in patients with erosive disease (Table 1). Unpredictable and frequent exacerbations are common, and in rare instances, continuous pain can be disabling.
Currently, there is no cure for oral lichen
planus. The large number of agents studied for
this disease reflects the inadequacy of any one
agent to control the symptoms in all patients.
Various oral health-care providers manage patients with oral lichen planus, including, but not
limited to, dentists, dermatologists, otolaryn-
1045-4411/93/S.50
© 1993by CRC Press, Inc.
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
141
TABLE 1
Epidemiology of Oral Lichen Planus
Ref.
Number of
patients
%
Males
%
Females
Age
%
Symptoms
Silverman et al. (1991)
Vincent et al. (1990)
Thorn et al. (1988)
Silverman et al. (1985)
Andreasen (1968)
214
100
611
517
115
29
24
33
23
35
71
76
67
11
65
54
64
53
52
50
73
79
48
69
NA
a
0
NA
17
3
0a
Erosive disease.
gologists, internists, and oncologists. The literature on this subject appears in many different
medical and dental specialty journals, making
access to the totality of information difficult on an
individual level. The purpose of this article is to
review and evaluate the many treatment modalities and measures that have been reported to be of
benefit in the management of patients with oral
lichen planus.
II. LICHENOID DRUG ERUPTIONS
There is a long and growing list of medications that have been implicated in the production
of lesions clinically and histologically identical to
oral lichen planus. These "drug-induced lichenoid
eruptions" always should be identified prior to
initiation of medical therapy. In general, one
should suspect any medication that patients take
as a possible cause of oral lichen planus. In particular, cardiovascular, antiarthritic, antimalarial,
and nonsteroidal anti-inflammatory drugs have
been most commonly linked with oral lichen
planus (Conklin and Blasberg, 1987). These same
medications may be implicated in lichenoid eruptions of the skin.
Many of the reported mucosal lichenoid reactions have not been well documented and substantiated, and thus many consider these to be rare
events (Scully and El-Kom, 1985).
Difficulties arise in suggesting drugs as the
cause of oral lichen planus because many medications may cause other types of mucosal lesions.
Some drug eruptions may be dose related, and
others may produce a lichenoid eruption only
142
%
Remission
with the first administration and not with repeated
challenges (Pennys et al., 1974). Although withdrawal of the implicated medications usually results in prompt clinical improvement, several
months may elapse before this is noted (Conklin
and Blasberg, 1987). Some authors maintain that
these drugs are not a cause of oral lichen planus,
rather that they reveal and amplify the disease
process in individuals who are genetically susceptible (Lacy et al., 1983). Regardless, because
there are a few well-documented and convincing
case reports of drugs causing oral lichen planus
(Potts et al., 1987), a thorough review of medications is warranted in all patients with this disease.
Lichenoid drug eruptions have received little
attention in almost all of the large series of patients reported with oral lichen planus. Perhaps
this entity has been overlooked, but more likely
its occurrence is rare.
I. CORTICOSTEROIDS
A. Topical
Undoubtedly, the most useful agent in the
treatment of oral lichen planus is corticosteroids.
Initially, only very weak preparations such as
hydrocortisone hemisuccinate pellets were available and were reported to be of some value in
erosive oral lichen planus (Cooke, 1965). However, Cawson (1968) subsequently showed that
only 3 of 18 patients receiving hydrocortisone
improved after 6 months of therapy. Triamcinolone
acetonide, a more potent steroid, was shown to be
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
useful as a long-lasting lozenge (Zegarelli et al,
1969) or in Orabase paste in a controlled study
(Rushton, 1962). However, only ulcerative lesions improved in these studies. Compacts of
triamcinolone acetonide bonded to molar teeth
produced salivary levels for 30 d in dogs (Deasy
et aL, 1989). However, when tested in five patients with oral lichen planus, only slight clinical
improvement was noted.
An aqueous suspension of triamcinolone
acetonide 0.1% was used recently as an oral rinse
in the treatment of 46 patients with symptomatic
oral lichen planus (Vincent et al, 1990). This
method proved to be effective, resulting in "complete relief in 27 patients. Although these results
most likely refer to improvement in patients' symptoms, no specific information is provided regarding the clinical improvement with this therapy.
Betamethasone valerate, an even more potent
anti-inflammatory agent, produced dramatic results in a number of controlled studies in patients
with oral lichen planus. In a double-blind study,
Cawson (1968) treated 30 patients with symptomatic oral lichen planus with betamethasone
(0.1 mg) pellets. In 8 patients, all lesions virtually
disappeared within 1 month, and during the same
period, 20 of 30 patients showed substantial improvement. Only two patients failed to respond to
this therapy. Similarly, Tyldesley and Harding
(1977) showed betamethasone valerate aerosol
fitted with a special intraoral adaptor was an excellent treatment in the majority of 23 patients
tested in a double-blind study. Greenspan et al.
(1978) confirmed the efficacy of both
betamethasone valerate aerosol and pellets in a
double-blind study, noting improvement in 17 of
19 patients.
In recent years, fluorinated and so-called
"superpotent" corticosteroids have been introduced
to treat various dermatologic cutaneous diseases.
Their topical anti-inflammatory activity, as assessed by the vasoconstrictor assay predicting
therapeutic efficacy, is higher than the aforementioned corticosteroids. Not surprisingly, their use
for oral vesiculoerosive diseases, including oral
lichen planus, has become increasingly popular.
Lozada (1980) studied the topical application
of fluocinonide 0.5% in an adhesive base (a fluorinated corticosteroid) in the treatment of various oral vesiculoerosive diseases. In this study,
67 patients with oral lichen planus applied the
medication five to six times daily to their oral
lesions. Eleven patients participated in a doubleblind study; of these, six showed a complete response and five showed a partial response to
therapy. Similarly, in the open phase of the study,
29 of 56 patients showed a complete response,
and 27 showed a partial response. The clinical
types of oral lichen planus lesions were not specified, and a partial response was defined only as
"improvement in signs or symptoms," making
these results difficult to interpret. Silverman et aL
(1991) reported that all of 22 patients treated with
fluocinonide ointment in an Orabase paste
achieved approximately 60% improvement in
symptoms after 2 weeks. Fluocinonide gel and
ointment have proven to be standard, first-line
therapies in the treatment of oral lichen planus
(Plemons et al, 1990).
Lozada-Nur and Huang (1991) studied nine
patients with erosive oral lichen planus. Patients
applied clobetasol propionate 0.025% in an
Orabase paste three times daily to their lesions.
This topical corticosteroid ranks as one of the
most potent preparations available worldwide. In
this open trial, five of nine patients had a complete response after 1 month of therapy. Two
patients improved by 50%, and two patients
showed absolutely no response. This trial was
open, and furthermore patients were maintained
on their systemic medications, including
prednisone and azathioprine, while using the topical corticosteroid. Conklin and Blasberg (1987)
also have advocated the use of clobetasol in severe cases of oral lichen planus, although controlled studies and its safety have not been documented.
Topical corticosteroids appear to be safe when
applied to mucous membranes. Lehner and Lyne
(1969) measured the plasma cortisol levels before
and after topical application of corticosteroids in
patients with oral diseases. There was no adrenal
suppression in 17 patients who applied 0.4 mg of
betamethasone valerate daily to their oral lesions
for several months. Triamcinolone acetonide in
doses up to 480 mg during several months was
found to produce no adverse effects, but plasma
cortisol levels were not measured (Kutcher et al.,
1966). Recently, Plemons et al (1990) definitively established the safety of fluocinonide gel in
143
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
patients who applied 1.5 g daily to oral lichen
planus lesions for 3 weeks. In this study, there
was no adrenal suppression, as assessed by measurements of serum and urine cortisol levels in
patients with erosive lichen planus as well as a
control group. Nevertheless, the prolonged use of
topical steroids on oral mucous membrane lesions
necessitates careful and frequent follow-up examinations. The potential for adrenal suppression
may be enhanced by the chronic application of a
potent corticosteroid to an already compromised
mucosal barrier in erosive disease. Furthermore,
not all topical corticosteroids are safe. Topical
application of betamethasone disodium phosphate
to oral lesions caused adrenal suppression in eight
of ten patients (Lehner and Lyne, 1969). The use
of betamethasone valerate aerosol in the form of
Valisone also can be harmful or fatal when applied to mucous membranes (Beckman, 1981).
The prolonged use of topical corticosteroids
invariably will result in secondary candidiasis in
some patients. Cawson (1968) reported its occurrence in 4 of 30 oral lichen planus patients treated
with betamethasone valerate. Candidiasis in the
oral cavity was developed by 3 of 24 patients
treated with topical clobetasol. As many as 31%
of patients treated with triamcinolone acetonide
suspension developed secondary candidiasis
(Vincent et al, 1990). This high incidence most
likely is the result of active corticosteroid being in
prolonged contact with the entire oral cavity and
not limited to the oral lichen planus lesions. In
patients requiring the chronic use of potent corticosteroid preparations, candidal cultures should
be performed routinely prior to therapy and periodically thereafter.
Despite the encouraging results obtained with
betamethasone valerate and other preparations,
topical corticosteroids do not alleviate the pain or
heal ulcerations in all patients. In fact, in one
study, 23 of 33 patients treated with topical or
systemic corticosteroids reported no symptomatic
change, and 8 reported only slight improvement
(Vincent etaL, 1990). Although these results seem
excessively incongruous with other published studies, they suggest that topical corticosteroids are
not universally effective. Even clobetasol propionate, the most potent of all topical corticosteroids, failed to produce any benefit in two of nine
patients treated for oral lichen planus (LozadaNur and Huang, 1991).
It is unclear why the response to topical corticosteroid therapy is so variable. Undoubtedly,
the frequency of application of topical corticosteroids makes compliance difficult because optimal effects are not achieved unless they are applied between five and ten times daily (Silverman
et aL, 1991). Additionally, the elderly, who comprise a significant portion of patients afflicted
with oral lichen planus, may find it technically
difficult to apply medication to all locations of the
oral cavity. Compounding the problem of achieving contact between the medication and the affected areas is the difficulty of adherence to moist
mucous membranes that are dislodged easily.
Zegarelli (1987) circumvented this problem by
prescribing a method whereby custom trays were
fabricated to prolong contact of topical corticosteroids with oral mucosa. This was not dissimilar
to the method reported by Aufdermorte et al.
(1985) for the treatment of mucous membrane
pemphigoid. It is obvious, however, that adjuvant
or alternative therapy is required for a select group
of patients with symptomatic oral lichen planus
unresponsive to topical therapy.
B. Intralesional
Sleeper (1967) found topical corticosteroids
to be of limited value in the treatment of lichen
planus and supplemented therapy with
intralesional triamcinolone acetonide suspension.
In his short but detailed account, seven patients
received 5 to 7 mg of triamcinolone injected into
lesions. All patients experienced relief of symptoms within 2 weeks; three showed complete healing of the lesions, and the remaining four showed
dramatic improvement clinically. The efficacy of
depot steroids (methylprednisolone acetate 40 mg/
ml) in the treatment of five patients with erosive
disease was documented by Ferguson (1977).
Within 1 week, four of five patients demonstrated
complete healing of their lesions. The benefits of
intralesional corticosteroids are now well known
and described (Randall and Cohen, 1974). Dusek
and Frick (1982) recommend a 5% mixture be
made with lidocaine to lessen the pain of the
injections.
Zegarelli (1983) combined the use of topical
and weekly intralesional corticosteroids in seven
patients. After 3 weeks, five patients were graded
144
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
as having 100% clinical improvement. Furthermore, in most cases, a remission of several months
was noted; recurrences were milder than the original disease state and were managed with topical
agents alone.
There are few contraindications to intralesional
corticosteroids. Frequent injections, especially
with depot steroids, may result in an unwanted
significant systemic dose. Atrophy of tissue and
secondary candidiasis are potential complications
that may occur locally. It is usually difficult to
inject sufficient quantities into gingival oral lichen planus lesions (Zegarelli, 1983). Three to
four weekly or biweekly treatments of intralesional
triamcinolone acetonide in doses of 5 to 10 mg/ml
appear to be a safe and practical method of supplementing therapy for erosive oral lichen planus.
C. Systemic
Although double-blind controlled studies have
not been performed with systemic corticosteroids
in the treatment of oral lichen planus, their frequent use in this disease attests to their efficacy.
The use of systemic corticosteroids should be
reserved for acute exacerbations. They most often
are used in combinations with topical corticosteroids, as their effects are immediate and can be
maintained with topical agents. However, their
use does not alter the natural course of oral lichen
planus. In fact, in the study by Silverman et al.
(1991), a much higher percentage of patients
achieved a symptom-free state with topical corticosteroids alone than with either systemic or a
combination of systemic and topical corticosteroids. Vincent et al. (1990) did not show a significant improvement with the combination of systemic and topical corticosteroids over topical corticosteroids used as a single agent. Zegarelli (1983)
compared various regimens of corticosteroids utilizing combinations of topical, injectable, and
systemic therapy. A slightly greater improvement
was noted when all three modalities were used in
individual patients. However, as expected,
candidiasis developed when combination therapy
was employed.
Various dosage regimens of systemic corticosteroids have been documented (Randall and
Cohen, 1974; Welton, 1969). In general, doses of
30 to 60 mg of prednisone, depending on the
severity of the disease, are administered once daily
for a 2 to 3 week period. The prolonged use of
systemic corticosteroids is limited by their inherent toxicity and should be employed only in extraordinary circumstances. Adverse effects are
common even when a short 2-week course of
therapy is administered (Lozada et al., 1984).
These most commonly include gastrointestinal
upset, mood alteration, polyuria, insomnia, and
shakes (Silverman et al., 1985). Few patients
develop significant changes in blood pressure or
blood glucose levels. However, these need to be
monitored with high doses or prolonged use. There
are medical contraindications that preclude the
use of systemic corticosteroids, necessitating physicians' consultation prior to the initiation of this
medication.
In summary, many but not all patients can be
managed with corticosteroids. Topical therapy
should be maintained until symptoms and clinical
findings improve. The decision to use cream, gel,
or ointment is based on the practitioner's personal
preference. Some may argue that gels tend to
sting and burn, whereas ointments do not; however, gels adhere to the oral mucosa more easily
than ointments. Regardless of shortcomings, they
are both effective. Ulcerations that do not respond
to topical agents can be injected with corticosteroids. Systemic corticosteroids should be reserved
for acute exacerbations characterized by multiple
ulcerations or widespread disease. Prolonged use
of any of the above modalities without supervision will result in undesirable systemic effects
and adverse local effects including candidiasis
and atrophy.
IV. RETINOIDS
The use of retinoids for the treatment of oral
lichen planus was first reported in 1973 by both
Gunther and Ebner et al. In these open, uncontrolled trials, vitamin A acid was applied locally
to white, reticulated lesions with good results.
Patients were asymptomatic and did not have erosive disease in these studies.
The most widely available and employed topical retinoid for the treatment of oral lichen planus
is tretinoin, a metabolite of vitamin A. Sloberg et
al. (1979) tested this agent in a mucosal adhesive
base at a concentration of 0.1% in 23 patients
145
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
with oral lichen planus. Although a total of 17
atrophic and erosive lesions were present in 23
patients, it is unclear how many patients actually
were aware of these lesions or how many had
discomfort associated with their disease. Furthermore, clinical results were graded as "improved",
"no change", or "worse", with improvement being defined as 50% healing of lesions. After 2
months of therapy in this open trial, 71% of atrophic and erosive lesions "improved", whereas the
remaining 29% of lesions were "unchanged" or
"worse." Side effects were limited to increased
soreness and redness, especially in erosive lesions. Relapses were common within 3 months of
discontinuing therapy. Interestingly, the authors
point out that a 0.05% concentration of tretinoin
had been used in a pilot study with unsatisfactory
results. High doses and frequent applications (four
times daily) were needed to achieve any response
with this therapy.
Giustina et al. (1986) published their results
with 0.1 % isotretinoin gel in the treatment of 20
patients with oral lichen planus. In this doubleblind study, patients applied the gel twice daily
for 2 months and were evaluated clinically on a
more universal five-point scale. Reticular and
plaquelike lesions improved or resolved with active therapy, whereas erosions, which diminished
in size, persisted the entire 2 months. In six patients with symptoms, five had complete resolution with therapy. All patients who received placebo improved only after switching to therapy
with active medication. Systemic levels of
isotretinoin were below detectable limits, and local side effects were few or nonexistent.
Isotretinoin gel currently is not available in the
U.S.; however, it is awaiting FDA approval for
use in acne vulgaris.
Handler (1984), in a "letter to the editor", was
the first to report beneficial results with systemic
isotretinoin for oral lichen planus. The recommended dose of isotretinoin for cystic acne is in
the range of 1 to 2 mg/kg/d. Handler used 0.25
mg/kg/d, minimizing side effects and observing
"excellent" results in seven patients after 2 months.
Staus and Bergfeld (1984) also supported the use
of low-dose isotretinoin in their report of a single
patient who was unresponsive to various treatments but showed clearing of erosions with
isotretinoin. Two patients with refractory erosive
oral lichen planus responded to isotretinoin at
doses of 0.5 to 1.0 mg/kg/d (Woo, 1985). Recurrent lesions developed after treatment was discontinued, however.
Camisa and Allen (1986) studied six patients
with oral lichen planus during 8 weeks of treatment with isotretinoin. Although no patient completely cleared in this study, all patients were
reported to have statistically significant improvement of symptoms. Four of the five evaluable
patients improved clinically, yet the authors concluded the isotretinoin was of minimal benefit in
this small study.
It is impossible to reach any definitive conclusions regarding the efficacy of isotretinoin in
the treatment of oral lichen planus. Controlled
studies have not been performed, and the open
trials that have been described consist of a handful of patients with few clinical details. This is
quite unfortunate in light of the beneficial results
obtained with topical isotretinoin in a doubleblind study. Furthermore, based on the histologic
features reported with topical isotretinoin in oral
lichen planus, including antikeratinization and
immunomodulation (Regezi et al., 1986), one
would expect similar or better results with systemic use of this medication.
The systemic use of vitamin A acid has beneficial results in oral lichen planus, but its toxicity
and side effects limit its use (Stuttgen, 1975).
Etretinate (RO 10-9359), a vitamin A analogue
with a better therapeutic index than vitamin A,
was first reported to be efficacious in the treatment of oral lichen planus by Schuppli (1978).
Ten patients, many of whom had erosive oral
lichen planus, were treated in an open trial with
50 mg of etretinate. After 2 to 3 weeks of therapy,
the dosage was reduced to 25 mg. Eight patients
had such "good effects" that the authors concluded that this therapy should be the first drug of
choice in the management of erosive lichen planus.
This open trial led Hersle et al (1982) to
conduct a double-blind study with etretinate.
Twenty-eight patients participated in a 2-month
study. All patients receiving etretinate, at an
average dose of 0.98 mg/kg/d, improved significantly, compared with only 5% of patients who
received placebo. Specifically, 12 or 14 atrophic
and erosive lesions responded to therapy. Unfortunately, a very inadequate subjective index was
146
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
used, defining a beneficial response as a 50% or
more reduction in erythema or erosion size.
Additionally, at this dose of etretinate, side effects were common, necessitating six patients
to withdraw early from the study. Three months
after the conclusion of the study, 68% of
patients developed a recurrence of their disease.
Despite these shortcomings, Hersle showed that
etretinate was a viable alternative to corticosteroids in refractory cases. In a subsequent study by
the same group, a lower dose of etretinate (0.6
mg/kg/d) was used in an attempt to minimize side
effects (Sloberg et al., 1983). After 2 months of
therapy, 81% of patients improved clinically, and
16 of 24 patients had resolution of pain. Side
effects of retinoids, including dryness and hair
loss, were common but usually mild, with only
one patient withdrawing early from the study.
After completing this 2-month study, patients were
enrolled in a maintenance regimen and received
either etretinate at 0.3 mg/kg/d or topical tretinoin
0.1% for 4 more months. In both groups, clinical
and symptomatic improvement was maintained
in more than two thirds of patients. The toxicity
of etretinate in the treatment of cutaneous disorders is dose related (Peck, 1980). This study demonstrated that adverse effects can be minimized
with low doses in the treatment of oral lichen
planus.
The subjective evaluations of the disease regression used in all of the above studies are vague,
making conclusions difficult to reproduce or validate.
Gorsky and Raviv (1992) recently reported
their results with etretinate as a first-line therapy
for oral lichen planus. Six patients received 75 mg
daily for 2 months in this open trial. Using a more
specific and detailed evaluation index, these authors showed that all patients improved in clinical
signs and symptoms using etretinate. Specifically,
erosive lesions disappeared, unlike in Sloberg's
study or in other studies utilizing topical retinoids.
Half of the patients achieved an asymptomatic
stage after the study, with only one patient withdrawing before completion because of adverse
effects. These results support Sloberg's conclusions and the efficacy of etretinate in the treatment of oral lichen planus.
As with isotretinoin, conflicting reports have
limited the use of etretinate in the management
of oral lichen planus. Ferguson et al. (1984)
published negative results after treating ten patients having erosive oral lichen planus with 75
mg of etretinate for 8 weeks. Two patients were
withdrawn after only 1 month because of unacceptable discomfort, and only one patient completed 8 weeks of the full dose of 75 mg daily.
In general, there was minimal improvement in
the degree of patient discomfort, and ulcerations
remained unchanged. Side effects were prominent and included cheilitis, pruritus, desquamation of hands and feet, paronychia, and hair loss,
all well-known side effects of vitamin A analogues. Others who have used etretinate for oral
lichen planus have reported similar findings to
those of Ferguson (Conklin and Blasberg, 1987).
Based on the above studies, the use of etretinate
should be reserved for refractory cases unresponsive to corticosteroids. Its use requires careful monitoring of hematologic and biochemical
laboratory values, including triglycerides and
liver transaminases, before and during therapy.
The side effects encountered with etretinate are
common and cumulative, requiring administration by an oral health care provider familiar with
its actions. Etretinate does not alter the natural
course of oral lichen planus. Both Hersle et al.
(1982) and Gorsky and Raviv (1992) reported
relapses shortly after discontinuing etretinate.
High doses (1 mg/kg/d) invariably result in side
effects unacceptable to patients, and very low
doses, as in Ferguson's study, probably are of
minimal value. Although no studies have been
published, the author has found the combination
of topical corticosteroids or topical retinoids and
a medium dose of etretinate (0.5 mg/kg/d) to be
beneficial.
Temarotene is a new retinoid analogue whose
use does not result in the undesirable side effects
of hypervitaminosis A. In an open pilot study,
nine patients with oral lichen planus were treated
for 1 month to 1 year with varying doses (Bollag
and Ott, 1989). Only one patient failed to respond, and all others showed either complete or
near complete remission. Relapses after discontinuing therapy were few, and erosive lesions
healed but required more than 4 to 6 months of
therapy. Double-blind studies are in progress to
evaluate the efficacy and optimal dose of this
promising new medication.
147
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
V. GRISEOFULVIN
The controversies in the management of oral
lichen planus are not limited to the use of systemic retinoids. Since the first report of
griseofulvin treatment of oral lichen planus appeared in the literature, the effectiveness of this
medication has been debated in journals and at
conferences. Sehgal et al. (1972) reported encouraging results using griseofulvin therapy in
cutaneous lichen planus in a double-blind study.
In their "discussion" section, these authors remarked that mucous membrane lesions responded
to therapy, but to a lesser degree than cutaneous
lesions. Massa and Rogers (1984) in a retrospective study examined the files of 11 patients with
oral lichen planus treated with griseofulvin. Of
these, 6 patients showed marked improvement or
complete remission within 3 weeks to 3 months
of therapy with daily doses of 500 mg or greater.
Of the respondents, three patients were treated
concomitantly with topical corticosteroids. In a
second group of subjects studied, 15 patients with
lichen planus exhibited both cutaneous and oral
lesions. Of these, only four patients showed improvement of their oral lesions with griseofulvin.
The use of griseofulvin in oral lichen planus
was supported by Aufdermorte etal. (1983). Three
cases of severe oral erosive lichen planus were
treated with 1 g of griseofulvin daily. One patient
had complete healing of erosions and complete
remission after 8 weeks of therapy. A second
patient showed a more rapid response by 3 weeks
and complete remission by 10 weeks. A third
patient showed marked improvement but persistent erosions after 10 weeks of therapy. Sustained
remissions were obtained in two patients for 9
and 15 months, respectively, after discontinuation
of griseofulvin. These preliminary results never
have been confirmed in double-blind studies. In
fact, since this initial report describing the dramatic results of this therapy, only two additional
reports have appeared, and both conflict with the
above findings.
Bagan et al. (1985) treated seven patients
with 1 g of griseofulvin daily for 2xli months.
Unlike the previous study by Massa and Rogers
whereby patients were examined every 3 months,
in this prospective study, patients were evaluated
weekly for the first month and biweekly thereafter. No improvement was seen in any patient, and
148
in four patients the condition worsened. Furthermore, one patient who was treated for 2 years
with griseofulvin for an unrelated fungal infection showed no improvement in his oral condition.
Naylor (1990) also failed to show any benefit
in four patients with erosive oral lichen planus
treated with griseofulvin. Until double-blind studies are performed confirming its efficacy, the use
of griseofulvin in the treatment of oral lichen
planus is unwarranted.
VI. CYCLOSPORINE
The etiology of lichen planus is unknown,
although various theories have been suggested.
The one that has gained the most acceptance in
recent years is based on an immunologic-mediated process. The histological changes characteristic of oral lichen planus involve a lichenoid
tissue reaction featuring subepithelial T lymphocyte infiltrate (De Panfilis et al., 1983). Damage
to the basement membrane is mediated by the
production of lymphokines such as Interferon
gamma by activated T lymphocytes (Takeuchi et
al., 1988). This molecule induces the expression
of intracellular adhesion molecule 1 and HLADR on the surfaces of keratinocytes. The interaction of keratinocytes via these molecules with
activated lymphocytes is thought to lead to the
destruction of the basement membrane observed
histologically (Farthing and Cruchley, 1989).
Cyclosporine is a potent immunosuppressant
that has been used in the treatment of graft rejection as well as in a host of dermatologic disorders.
Its mechanism of action is unknown; however, it
selectively inhibits the proliferation and function
of T lymphocytes, thereby reducing the production of lymphokines such as Interferon gamma
(Wagner, 1983).
The use of systemic Cyclosporine has been
shown to be of benefit in cutaneous lichen planus,
resulting in sustained remissions (Pigatto et al.,
1990), but its use requires careful monitoring.
Adverse effects of systemic Cyclosporine on
renal function negate its long-term appropriateness in chronic disorders such as oral lichen
planus.
Frances et al. (1988) are credited with the
first report on the use of topical Cyclosporine for
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
erosive oral lichen planus. This novel approach
resulted in improvement in all four patients treated
with 100 mg of topical Cyclosporine for 1 month.
Two patients showed 80% improvement in erosions, and two others showed a 40% reduction.
Although Cyclosporine blood levels were recorded, these were relatively low, and abnormal
laboratory values were nonexistent. Balato et al.
(1989) confirmed Frances' results with topical
Cyclosporine. Seven patients with oral lichen
planus, all of whom had erosive disease, were
treated topically for 4 months. Patients received
100 mg daily for the first 2 months and 50 mg
daily thereafter. Four of seven patients showed
complete healing of all erosions, and the remaining three patients showed 40 to 80% re-epithelialization of all erosions. Shortly thereafter, Eisen et
al. (1990) utilized Cyclosporine as a "swish-andspit" medication in an open trial of six patients.
Doses of Cyclosporine were considerably higher
than in previous studies, with patients swishing
500 mg three times daily for 8 weeks. All patients
showed improvement in atrophic and erosive lesions, with transient burning of the mucosal surfaces noted as the primary side effect. Whole
blood Cyclosporine levels were either undetectable or low. Biopsies performed in four patients
prior to the study showed keratinocytes expressing intracellular adhesion molecule 1 and HLADR on their surfaces. After 8 weeks of
Cyclosporine, intracellular adhesion molecule 1
was virtually undetectable, and HLA-DR was
moderately reduced, consistent with the mechanistic actions of Cyclosporine.
Recently, a double-blind analysis was performed with Cyclosporine swish in 16 patients
(Eisen et al., 1990). Patients who received
Cyclosporine swished 500 mg of medication for
5 min, three times daily, for 8 weeks. Those who
received placebo had no change or minimal improvement after 8 weeks, whereas patients who
received active medication showed marked improvement in atrophic, erosive, and reticular lesions. Pain, present in all patients prior to the
study, was improved significantly or resolved in
all patients receiving Cyclosporine. Although
whole blood Cyclosporine levels were low in nine
patients, seven patients had undetectable blood
levels. There were no systemic adverse effects,
and laboratory values that were measured throughout the study remained unchanged. Clinical im-
provement and global scores were assessed on a
scale of-1 to 3: -1 representing worsening; 0, no
change to minimal improvement (0 to 20%); 1,
moderate improvement (20 to 49%); 2, marked
improvement (50 to 80%); and 3, complete improvement (81 to 100%). This subjective scale
allows for a more precise clinical evaluation than
we have seen in the previous literature and has
been adapted and modified by other authors
(Silverman etal, 1991; Gorsky and Raviv, 1992).
Clinical remissions of 3 to 8 month durations
were noted in 8 of 12 patients. Interestingly, at the
end of therapy, biopsies obtained from patients
receiving active medication showed exceedingly
high Cyclosporine levels similar to levels reported
in psoriatic lesions following treatment with systemic Cyclosporine at high doses (14 mg/kg/d).
This suggests that Cyclosporine, which was absorbed at high levels by the oral mucosa, was
responsible for a local immunologically mediated
process.
Unfortunately, the use of Cyclosporine in treatment of oral lichen planus is limited by its high
cost. In the double-blind study described above,
1500 mg of Cyclosporine was used daily at an
average cost of $60 to $70 per day. This is exceedingly high in comparison with 25 to 50 cents
per day for the use of topical corticosteroids. Although it was speculated that low doses (and thus
lower cost) would be effective based on the studies of Balato et al. (1989) and Frances et al
(1988), who used 100 mg of Cyclosporine daily,
this premise was negated by Ho and Conklin
(1991). In their study, four patients who swished
600 mg of Cyclosporine daily showed no improvement after 4 months of therapy. Levell et al.
(1991) also reported the lack of efficacy of topical
Cyclosporine in five patients who used
Cyclosporine as a rinse for 4 weeks. Only five
patients completed the study, and four of these
showed only a modest degree of improvement.
These results conflict with those from three open
studies and one double-blind study attesting to
the efficacy of topical Cyclosporine. Some oral
health-care providers maintain that Cyclosporine
swish is effective only if systemic blood levels
are achieved; however, many others have found
the topical preparation to be clinically effective
without detectable Cyclosporine blood levels (personal communication). Further research should
be aimed at improving the vehicle to enhance
149
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Cyclosporine penetration resulting in a lower effective dose, thus, a lower cost.
VII. ALTERNATIVE THERAPIES
Although the therapy of oral lichen planus
has improved over the past decade with the
development of potent topical corticosteroids and
vitamin A analogues, new therapeutic modalities
are continuously undergoing investigation. The
following section highlights some of these advances.
A. Phenytoin
Phenytoin is an anticonvulsive medication that
has been shown to promote wound healing and
modulate immunologic functions. In an open trial
involving 30 patients with cutaneous lichen planus,
4 patients who demonstrated oral lesions were
noted to have complete healing, and 2 others did
not show any significant response (Bogaert and
Sanchez, 1990). Further studies are under way to
evaluate this treatment modality.
B. Antibacterial and Antiviral Agents
In a series of patients with desquamative gingivitis, six subjects with oral lichen planus were
treated with doxycycline monohydrate at 100 mg
daily for 3 weeks (Ronbeck et al, 1990). This
derivative of tetracycline produced only modest
results. One patient improved dramatically, three
patients improved slightly, and two patients were
either unchanged or worse after the therapy. The
benefits of this drug most likely are due to its antiinflammatory action and not its antibacterial activity. A bacterial etiology was suggested for lichen planus by early investigators; however, electron microscopy and cultures failed to reveal bacteria (Whitten, 1970). Furthermore, antimicrobial
agents, including tetracycline, have been of no
value in the treatment of oral lichen planus.
Doxycycline has yet to be tested in the treatment
of the more standard forms of oral lichen planus.
A group of patients with oral lichen planus
exhibiting the desquamative gingivitis form was
tested with systemic dapsone. This sulfone, which
has been used for a variety of dermatoses as well
as leprosy, also did not produce remarkable results (Mathews etal, 1989). Specifically, of seven
patients treated, one showed complete recovery,
three showed mild improvement, and three showed
no response.
In two case reports, dapsone was shown to be
of benefit in the erosive lesions of oral lichen
planus (Beck and Brandrup, 1986; Falk et al,
1985). Improvement requires prolonged treatment
and careful evaluation with laboratory investigations, as dapsone is known to cause adverse reactions. Hemolysis, nausea, and headaches caused
by dapsone may outweigh any likely moderate
benefits in the treatment of oral lichen planus
(Editorial, 1990).
Histologic examination of lesions from patients with oral lichen planus occasionally exhibits dysplasia. Ten patients with oral lichen planus
exhibiting dysplastic features and eight with erosive disease were treated with topical human fibroblast Interferon-B in a water-soluble gel preparation twice weekly (Sato et al, 1985). After ten
treatments with this antiviral preparation, complete remission of pain and clinical signs was
noted, with adverse reactions limited to some local erythema and swelling. Hyperkeratotic lesions
did not respond as well as erosive lesions. Unfortunately, no further studies have been reported
since this pilot study was published several years
ago.
C. Azathioprine
Azathioprine is a potent immunosuppressive
agent with well-known adverse effects, including
bone marrow suppression. Additionally, long-term
use of this medication may increase the risk of
developing internal malignancies (Moschella,
1977). In combination with prednisone, four patients with oral lichen planus "responded slowly"
to this therapy, with three of four patients achieving a partial clinical remission (Lozada, 1981).
Doses of azathioprine were low (50 mg/d), and
the duration of treatment was short (1 to 2 months).
Nevertheless, the minimum effective dose of
prednisone was reduced when given in combination with azathioprine. Silverman et al. (1991)
also used azathioprine in doses of 50 to 100 mg/d
150
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
for a 2-week period in combination with systemic
corticosteroids in the treatment of five patients
with oral lichen planus. Although all patients responded, there was no difference in the response
of this small group compared with patients receiving either systemic prednisone with topical
corticosteroids or systemic prednisone alone. The
minimal benefit obtained is undoubtedly due to
the relatively short 2-week treatment period with
azathioprine, which usually requires months before its maximum effects are achieved (Van Dijk
and Van Velde, 1973; Greaves et ai, 1971).
D. Ultraviolet Light
One of the more common treatment modalities for cutaneous lichen planus, as well as other
dermatoses, involves photochemotherapy with
psoralens and long-wave ultraviolet-A (PUVA).
Jansen et ai (1987) modified a unit intended for
light-cured dental composite fillings to administer ultraviolet-A in the oral cavity. Patients ingested psoralens, a photosensitizer, prior to the
administration of ultraviolet-A and were irradiated at intervals of 2 to 3 d, similar to treatment
for skin disorders. All eight patients with refractory and symptomatic atrophic and erosive oral
lichen planus responded to therapy. The number
of treatments per patient ranged from 4 to 12, and,
even after treatment was discontinued, patients
continued to improve clinically. At a 6-month
follow-up, five of eight patients had complete or
marked resolution of their disease. The same group
reported findings in a larger series of 17 patients
treated with mouth PUVA with similar results
(Lehtineneftf/., 1989).
Patients exposed to PUVA are known to have
an increased risk of developing squamous cell
carcinomas (Forman et ai, 1989). This is especially troublesome because many consider oral
lichen planus to be a premalignant condition
(Scully and El-Kom, 1985; Holmstrup etaL, 1988).
One would certainly need to evaluate extensively
the safety of a method that initiates or promotes
cancer in the treatment of an already premalignant
oral condition.
A similar new treatment for oral lichen planus
was described by Chen (1989). Weekly treatments
of ultraviolet-A without systemic or topical photosensitizers were received by 35 patients. After
eight treatments, 87% of patients were either cured
or had significantly improved clinically, and resolution of pain also was achieved in most patients.
No malignancy was noted during 5 years of observation following irradiation. Interestingly, ultraviolet-A appears to be selectively efficacious
in the treatment of oral lichen planus because
there are no studies documenting its beneficial
effects in the treatment of cutaneous lichen planus
or psoriasis without the use of photosensitizers.
The efficacy of mouth PUVA and UVA in the
treatment of oral lichen planus supports the role
of the immune system in its pathogenesis, but
these treatments should be considered experimental.
E. Surgery
Cryosurgery is a well-known treatment modality familiar to dermatologists for the treatment
of many neoplasms. Tal and Rafkin (1986) reported their experience in treating a reticulated
lesion on the gingiva with cryosurgery. Although
treatment was for cosmetic reasons, the treatment
was successful, and keratinized lesions had not
reappeared at a 2-year follow-up examination. An
erosive lesion of lichen planus unresponsive to
conventional therapy also was treated by Loitz
and O'Leary (1986) with cryosurgery. The patient required hospitalization and general anesthesia, but complete resolution of symptoms was
seen within 1 week of therapy and healing of the
ulcer occurred by day 16. Many authors have
reported similar benefits with the use of
cryosurgery in the treatment of oral lesions and
oral lichen planus (Malurstrom and Leikomaa,
1980; Leopard and Poswillo, 1974). The use of
portable cryosurgical units in the office setting for
the treatment of erosive oral lichen planus has not
been studied. Cryosurgery results in tissue destruction and theoretically can cause erosions to
enlarge, necessitating close observation of the
patient's clinical status.
Surgical treatment of selected oral lichen
planus lesions also has been recommended as
described in four patients with symptomatic lesions that were excised (Emslie and Hardman,
1970). Vedtofte et al. (1987) excised dysplastic
lesions in five patients with oral lichen planus,
with minimal complications and no recurrences.
151
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
As an alternative to scalpel surgery, the CO2
laser was used to treat three patients with erosive
oral lichen planus (Frame et al, 1984). Although
re-epithelialization occurred by 4 to 6 weeks with
minimal wound contraction, two patients experienced recurrences. Horch et al. (1986) treated
seven patients with erosive oral lichen planus
with a CO2 laser, with only one recurrence noted
after 37 months of follow-up. The post-surgical
period resulted in minimal pain, and healing occurred without contractions.
The use of cryosurgery and CO2 laser surgery
may indeed be an adequate treatment of dysplastic lesions in oral lichen planus. However, their
use is limited in that tissue is destroyed and the
lesions cannot be completely examined histologically. Surgical excision of atrophic and erosive
lesions should never be thought of as a primary
method of treatment because oral lichen planus is
an inflammatory condition that will invariably
recur. Furthermore, trauma to the oral mucosa by
a surgical procedure may induce the formation of
new lesions at these sites (Katz et al, 1988).
association of oral lichen planus with cigarette
use; however, Murti et al (1986), studying an
Indian population, did. Additionally, the prevalence of oral lichen planus is higher in individuals
who chew tobacco or betel (Pindborg et al, 1972;
Daftary et al, 1980).
B. Plaque
VIM. ELIMINATING EXACERBATING
FACTORS
Dental plaque certainly could induce lesions
through a Kobner phenomenon, especially with
patients afflicted with gingival lichen planus. The
beneficial effects of optimal plaque control in
patients with oral erosive lichen planus were described by Erpenstein (1985). Holmstrup et al
(1990) also reported results of the effect of dental
plaque control on gingival lichen planus in 11
patients. With an intensive individual hygiene
program as the sole method of therapy for 1 year,
9 of 11 patients experienced improvement in symptoms. As expected, the resolution of pain corresponded to the decrease in plaque scores. Care
must be taken, however, with aggressive periodontal treatment and surgery that may result in
worsening of lichen planus, presumably by the
Kobner phenomenon.
A. Oral Habits
C. Stress
Among various cutaneous diseases, lichen
planus is known to be associated with the Kobner
phenomenon. By definition, damage or trauma to
clinically normal skin in patients with lichen planus
results in the development of new lesions at these
sites. This phenomenon may explain the frequency
of lichen planus erosions on the buccal mucosa
and tongue, which are especially prone to trauma.
In the management of oral lichen planus, it is
important to minimize irritants that may contribute to the Kobner phenomenon. For example,
rough dental restorations should be corrected.
Similarly, oral habits such as tongue and cheek
biting or lip chewing should be discouraged.
Cigarette smoking may act as an irritant as
well. The incidence of tobacco use, however, is
not greater in patients with oral lichen planus than
controls (Neumann-Jensen et al, 1977). Silverman
et al (1985) in a large series also demonstrated no
In his original description of patients with oral
lichen planus in 1869, Erasmus Wilson noted that
many of his patients experienced emotional stress
(Wilson, 1869). Since then, various conflicting
reports have been published regarding the link
between psychogenic factors and oral lichen planus.
In a study of 49 patients with oral lichen planus, an
association was found between erosive disease and
emotional stress, but none existed in patients with
asymptomatic oral lichen planus (Lowenthal and
Pisanti, 1984). Others also have reported a worsening of the disease in periods of stress (Hampf et al,
1987). However, Allen et al (1986), in their study
of 48 patients with oral lichen planus, showed no
association between emotional events and the disease process. Treatment should be tailored toward
the needs of the individual, especially if stress is
identified as an important precipitating factor in
the patient's oral condition.
152
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
D. Diet
the theory that dental restorations are a common
cause of this disease.
With widespread oral ulcerations, eating often is compromised due to pain and discomfort.
Poor and delayed wound healing can result from
an inadequate nutritional status, especially in elderly patients. Although vitamin deficiencies were
not found to be greater in patients with oral lichen
planus than in control subjects, correction of low
levels of vitamin B{ and B 6 resulted in clinical
and subjective improvement in the majority of
treated patients (Jolly and Nobile, 1977). Patients
not only should try to maintain adequate dietary
habits but also be instructed to avoid foods that
they find cause pain and exacerbate their disease.
E. Restorative Materials
There are reports that contact mucositis to
mercury and other restorative dental materials
may be significant in the etiology of oral lichen
planus. Finne et al (1982) patch-tested 29 patients with typical oral lichen planus, testing for a
contact allergy to dental materials. In 18 of these
patients (62%) an allergy to mercury was documented, whereas only 3.2% of patients were positive in a control group. Furthermore, in three of
four patients whose patch test was positive to
mercury and whose amalgams were removed and
replaced by other restorative materials, oral lesions healed within 1 year. These authors recommended performing patch testing on all patients
with oral lichen planus. Frykholm et al. (1969)
also suggested a contact mucositis to copper in
dental alloys as a cause of oral lichen planus.
Electrogalvanic effects of metals have been implicated as well (Banoczy et al, 1979; Holland,
1980). Even reactions to gold restorations have
been suggested although never substantiated
(Conklin and Blasberg, 1987).
There appears to be a small subgroup of patients who exhibit erosions clinically and oral
lichen planus histologically at sites adjacent to
worn and damaged restorations, especially amalgams. Even composite restorations may cause
lichenoid reactions (Lind, 1988). In such cases, it
may be worthwhile to replace these restorations.
Obviously, because edentulous patients exhibit
oral lichen planus, little support has been given to
F. Candida
When treating oral lichen planus, the role of
Candida always should be evaluated carefully.
As discussed earlier, treatment with topical,
intralesional, or systemic corticosteroids predisposes to secondary candidiasis, which necessitates monitoring patients with this therapy.
Studies have demonstrated that Candida is
present in roughly a third to half of all oral lichen
planus patients, a prevalence not statistically different from the normal population (Lundstrom et
al, 1984; Krogh etal, 1987; Simon and Hornstein,
1980). However, a moderate to heavy growth of
Candida, indicating colonization, was obtained in
29% of oral lichen planus patients but only 7% of
a control group (Lundstrom et al, 1984). Hatchuel
et al (1990) studied the prevalence of Candida in
185 biopsies of oral lichen planus patients. When
compared with a control group of 120 patients
showing only two infected cases, Candida infection was found in approximately 34% of the oral
lichen planus group. There was no difference
between ulcerative and nonulcerated lichen planus
cases. With respect to the biotype, Krogh et al
(1987) showed that Candida albicans was the
dominating species isolated from oral lichen
planus, although Saccharomyces cerevisiae and
C. pintolopesii also were found. The higher rate
of Candida may indicate a possible impairment in
cellular immunity in patients with oral lichen
planus.
In 17 of 18 oral lichen planus patients with
positive candidal cultures, antifungal treatment
with amphotericin B resulted in marked symptomatic improvement in 89% and clinical improvement in 94% of patients. Vincent et al
(1990), in a study of 100 patients with oral lichen
planus, reported secondary candidiasis in 31% of
symptomatic patients. Candida treatment often
resulted in resolution of pain and clinical improvement. These studies taken together demonstrate the need to constantly evaluate the role of
Candida in this chronic condition. A change in
the patient's clinical status or an acute exacerbation, especially in patients being treated with cor-
153
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
ticosteroids, should alert the practitioner to a potential secondary candidal infection.
The benefits of antifungal therapy in the
management of oral lichen planus have been accepted by most authors. Systemic ketoconazole at
doses of 200 mg daily for 2 to 3 weeks at the
initiation of therapy will augment the effects of
most therapies employed. Alternatively, one may
use topical antifungal therapy with equally good
results. Some do not use antifungal therapy unless
candidiasis is evident (Silverman et al, 1991).
IX. CONCLUSION
In patients with oral lichen planus who are
symptomatic, the primary goal of therapy is palliative. Although corticosteroids usually are effective when applied topically or given
intralesionally or systemically, there are potential
adverse effects locally in the oral cavity and internally. This is especially true with their chronic
use. Corticosteroids never have been compared in
trials with any other agent in the treatment of oral
lichen planus. This is of minor importance because there does not appear to be a more effective
agent available. However, because all patients
will not achieve symptomatic relief with corticosteroids, investigators have been compelled to study
newer topical and systemic medications. Topical
and systemic retinoids are effective in recalcitrant
cases, but these too are limited by their potential
toxicity. Only those familiar with the adverse reactions of systemic retinoids should administer
these drugs, which obviously limits their use. No
studies have been performed using combinations
of retinoids and corticosteroids. In practice among
dermatologists, this is a commonly employed regimen that probably demonstrates greater efficacy
than the use of either agent used alone. Newer
retinoids, such as temarotene, are being developed with fewer adverse reactions. Invariably,
these will be tested in the treatment of oral lichen
planus.
The demonstrated efficacy of Cyclosporine
and mouth PUVA is important in elucidating the
etiology of lichen planus, and both support the
role of the immune system in its pathogenesis.
Newer immunomodulators similar to Cyclosporine
are being developed, including FK 506, used in
the treatment of graft rejection. Such agents may
have fewer adverse reactions and already are being tested for dermatologic diseases such as psoriasis. Many of these may be employed for T-cellmediated diseases such as oral lichen planus.
In the management of patients with oral lichen planus, one should choose agents that result
in patient comfort with the fewest adverse effects.
Therefore, patient education is an integral component of treatment that should start prior to the
initiation of therapy. Patients often have unrealistic expectations of therapy, especially those with
widespread disease. All patients should be advised that their disease often will be lifelong and
characterized by unpredictable exacerbations and
remissions. Symptomatic relief can be achieved
in the majority of patients with many of the agents
discussed or, more often, with combinations of
these medications. Only rarely will patients require the prolonged use of systemic medications.
A complete clinical evaluation includes a review of any medications that may be a cause of
the disease. An overall assessment of the patient's
nutritional and emotional status as well as the
elimination of precipitating factors, including traumatic habits and damaged dental restorations, is
warranted. These may seem trivial and may rarely
be relevant; however, any benefits achieved are
significant when compared with a lifelong commitment to topical and systemic medications.
Despite studies that have shown that Candida
is not more prevalent in patients with oral lichen
planus, its role in causing exacerbations is undisputed. This is especially true in patients who have
been managed with chronic use of corticosteroids. One should always re-evaluate patients for
secondary candidiasis with cultures or smears,
regardless of the therapy employed. Empiric treatment with topical or systemic antifungal therapy
often will result in clinical improvement of the
lichen planus because many of these agents possess anti-inflammatory as well as antifungal effects (Cutsem et al., 1991).
All of the above medications and measures treat
an inflammatory reaction that has been elicited by
some unknown antigen. Thus, none is specific or
curative. More accurate treatment can be possible
only if the exact etiology is identified. Until then,
new treatments are welcomed not only for their
clinical benefit but also for clues they provide in
unraveling the pathogenesis of this complex disease.
154
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Almost without exception, the different treatments for oral lichen planus provoke controversy.
Conflicting reports about the efficacy of
griseofulvin, retinoids, Cyclosporine, and even
corticosteroids are easily found in the literature.
There is also no agreement about the effects of
eliminating drugs, restorations, psychologic stress,
tobacco, and Candida in this disease. This suggests that oral lichen planus is a heterogeneous
disorder, all forms having similar clinical and
histologic patterns. Many patients may be genetically susceptible to this condition with the appearance of clinical signs induced only by certain
stimuli. These include drug reactions, dental restorations, stress, and possibly numerous other
unknown factors. Even various diseases that have
been reported to be associated with lichen planus,
including diabetes, hypertension, gastrointestinal
diseases, and other autoimmune disorders, may
unmask oral lichen planus. Rigid criteria in classifying and identifying subgroups of patients with
oral lichen planus may lead to treatments that are
accepted uniformly and effective universally in
each form of oral lichen planus.
REFERENCES
Andreasen, J. O.: Oral Lichen Planus: A Clinical Evaluation
of 115 Cases. Oral Surg. Oral Med. Oral Pathol. 25:3141 (1968).
Allen, C. M , F. M. Beck, K. M. Rossie and T. J. Kavi:
Relation of Stress and Anxiety to Oral Lichen Planus.
Oral Surg. Oral Med. Oral Pathol. 61:44-46 (1986).
Aufdermorte, T. B., R. L. De Villez and D. R. Gieseker:
Griseofulvin in the Treatment of Three Cases of Oral
Erosive Lichen Planus. Oral Surg. Oral Med. Oral Pathol.
55:459-462 (1983).
Aufdermorte, T. B., R. L. De Villez and S. M. Parel: Modified Topical Steroid Therapy for the Treatment of Oral
Mucous Membrane Pemphigoid. Oral Surg. Oral Med.
Oral Pathol. 59:256-260 (1985).
Bagan, J. V., F. J. Silvestre, S. Mestre, C. Gisbert, A. Bermejo
and J. Agramont: Treatment of Lichen Planus with
Griseofulvin. Report of Seven Cases. Oral Surg. Oral
Med. Oral Pathol. 60:608-610 (1985).
Balato, N., S. De Rosa, S. Bordone and F. Ayala: Dermatological Application of Cyclosporine. Arch. Dermatol.
125:1430-1431 (1989).
Banoczy, J., B. Roed Peterson and J. Pindborg: Clinical and
Histologic Studies on Electrogalvanically Induced Oral
White Lesions. Oral Surg. OralMed. Oral Pathol. 48:319328 (1979).
Beck, H. I. and S. Brandrup: Treatment of Erosive Lichen
Planus With Dapsone. Acta Derm. Venereol. 60:366-367
(1986).
Beckman, B. I.: Valisone Aerosol Spray Contraindicated in
Mucous Membranes. J. Am. Ac ad. Dermatol. 4:233
(1981).
Bogaert, H. and E. Sanchez: Lichen Planus: Treatment of
Thirty Cases With Systemic and Topical Phenytoin. Int.
J. Dermatol. 29:157-158 (1990).
Bollag, W. and F. Ott: Treatment of Lichen Planus with
Temarotene. Lancet. 2:974(1989).
Camisa, C. and C. M. Allen: Treatment of Oral Erosive
Lichen Planus with Systemic Isotretinoin. Oral Surg.
Oral Med. Oral Pathol. 62:393-396 (1986).
Cawson, R. A.: Treatment of Oral Lichen Planus with
Betamethasone. Br. Med. J. 2:86-89 (1968).
Chen, H. R.: A Newly Developed Method for Treatment of
Oral Lichen Planus With Ultraviolet Irradiation. /.
Formosan Med. Assoc. 88:248-252 (1989).
Conklin, R. J. and B. Blasberg: Oral Lichen Planus. Dermatol.
Clin. 5:663-673 (1987).
Cooke, B. E. D.: Oral Ulceration as a Manifestation of Some
Dermatoses. R. Soc. Med. Proc. 58:455-459 (1965).
Cutsem, J. V., F. V. Gerven, G. Cauwenbergh, F. Odds and
P. A. J. Janssen: The Antiinflammatory Effects of
Ketoconazole. /. Am. Acad. Dermatol. 25:257-261 (1991).
Daftary, D. K., R. B. Bhonsle, R. B. Musti, J. J. Pindborg and
F. S. Mehta: An Oral Lichen Planus-Like Lesion in Indian Betel Tobacco Chewers. Scand. J. Dent. Res. 88:244245 (1980).
De Panfilis, G., G. Manara, P. Sansoni and F. Allegra: T Cell
Infiltrate in Lichen Planus: Demonstration of Activated
Lymphocytes Using Monoclonal Antibodies. /. Cutaneous Pathol. 10:52-58 (1983).
Deasy, P. B., A. E. Collins, F. M. Burke and D. B. Shanley:
In Vitro and In Vivo Evaluation of a Bondable Compact
for the Prolonged Delivery of Triamcinolone Acetonide
to the Oral Cavity in Patients with Lichen Planus. Pharm.
Acta Helv. 64:276-279 (1989).
Dusek, J. and W. Frick: Lichen Planus: Oral Manifestations
and Suggested Treatments. /. Oral Maxillofac. Surg.
40:240-244 (1982).
Ebner, H., P. Mischer and M. Raff: Lokal Behandlung des
Lichen Rubber Planus der Mundehlermhaut mit Vitamin
A Savre. Z. Hautkr. 48:735-740 (1973).
Editorial: Treatment of Oral Lichen Planus. Lancet. 336:913914 (1990).
Eisen, D., C. E. M. Griffiths, C. N. Ellis, B. J. Nickoloff and
J. J. Voorhees: Cyclosporin Wash for Oral Lichen Planus.
Lancet. 335:535-536 (1990).
Eisen, D., C. N. Ellis, E. A. Duell, C. E. M. Griffiths and J. J.
Voorhees: Effect of Topical Cyclosporine Rinse on Oral
Lichen Planus: a Double-Blind Analysis. N. Engl. J. Med.
323:290-294 (1990).
Emslie, E. S. and F. G. Hardman: The Surgical Treatment of
Oral Lichen Planus. Trans. St. Johns Hosp. Dermatol.
Soc. 56:43-44 (1970).
Erpenstein, H.: Periodontal and Prosthetic Treatment in Patients With Oral Lichen Planus. /. Clin. Periodontol.
12:104-112(1985).
155
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Falk, D. K., D. L. Latour and L. E. King: Dapsone in the
Treatment of Erosive Lichen Planus. / . Am. Acad.
Dermatol. 12:567-570 (1985).
Farthing, P. M. and A. T. Cruchley: Expression of MHC
Class II Antigens (HLA DR, DP, and DQ) by
Keratinocytes in Oral Lichen Planus. /. Oral Pathol.
Med. 18:305-309 (1989).
Ferguson, M. M.: Treatment of Erosive Lichen Planus of the
Oral Mucosa with Depot Steroids. Lancet. 2:771-772
(1977).
Ferguson, M. M., M. B. Simpson and N. Hammersley: The
Treatment of Erosive Lichen Planus with a Retinoid—
Etretinate. Oral Surg. Oral Med. Oral Pathol. 58:283287 (1984).
Finne, K., K. Goransson and L. Winckler: Oral Lichen Planus
and Contact Allergy to Mercury. Int. J. Oral Surg. 11:236239 (1982).
Forman, A. B., H. H. Roenigk and W. A. Caro: Long-term
Follow-up of Skin Cancer in the PUVA-48 Cooperative
Study. Arch. Dermatol. 125:515-519 (1989).
Frances, C , S. Boisnic, S. Etienne and H. Szpirglas: Effect
of the Local Application of Cyclosporine A on Chronic
Erosive Lichen Planus of the Oral Cavity. Dermatologica.
177:194-195(1988).
Frame, J. W., A. R. Das Gupta, G. A. Dalton and P. H. RhysEvans: Use of the Carbon Dioxide Laser in the Management of Premalignant Lesions of the Oral Mucosa. J.
Laryngol. Otol. 98:1251-1260 (1984).
Frykholm, K. O., L. Frithiofi, A. Fernstron, G. Moberger,
S. F. Blom and E. Bjorn: Allergy to Copper Derived from
Dental Alloys as a Possible Cause of Oral Lesions of
Lichen Planus. Acta Derm. Venereol. 49:268-281 (1969).
Giustina, T. A., J. C. B. Stewart, C. E. Ellis, J. A. Regezi, T.
Annesley, T. Y. Woo and J. J. Voorhees: Topical Application of Isotretinoin Gel Improves Oral Lichen Planus.
/. Arch. Dermatol. 122:534-536 (1986).
Gorsky, M. and M. Raviv: Efficacy of Etretinate (Tegison)
in Symptomatic Oral Lichen Planus. Oral Surg. Oral
Med. Oral Pathol. 73:52-55 (1992).
Greaves, M. W., J. L. Burtone, J. Marks and R. P. Dawber:
Azathioprine in Treatment of Bullous Pemphigoid. Br.
Med.J. 1:144-145 (1971).
Greenspan, J. S., C. M. Yeoman and S. M. Harding: Oral
Lichen Planus: A Double-Blind Comparison of Treatment with Betamethasone Valerate Aerosol and Pellets.
Br. Dent. J. 144:83-84 (1978).
Gunther, S. W.: Vitamin A Acid in Treatment of Oral Lichen
Planus. Arch. Dermatol. 107:277 (1973).
Hampf, B. G., M. J. Malstron, V. A. Halberg, J. A. Hannula
and J. Vikkula: Psychiatric Disturbance in Patients with
Oral Lichen Planus. Oral Surg. Oral Med. Oral Pathol.
63:429-432(1987).
Handler, A. L.: Isotretinoin for Oral Lichen Planus. /. Am.
Acad. Dermatol. 10:674 (1984).
Hatchuel, D. A., E. Peters, J. Lemmer, J. J. Hille and W. T.
McGaw: Candidal Infection in Oral Lichen Planus. Oral
Surg. Oral Med. Oral Pathol. 70:127-175 (1990).
Hersle, K., H. Mobacken, K. Sloberg and H. Thilander:
Severe Oral Lichen Planus: Treatment with an Aromatic
Retinoid (Etretinate). Br. J. Dermatol. 106:77-80 (1982).
Ho, V. C. and R. J. Conklin: Effect of Topical Cyclosporine
on Oral Lichen Planus. N. Engl. J. Med. 325:435 (1981).
Holland, R.: Galvanic Currents Between Gold and Amalgam. Scand. J. Dent. Res. 988:269-272 (1980).
Holmstrup, P., A. W. Schiotz, D. Hyg and W. Westergaard:
Effect of Dental Plaque Control on Gingival Lichen Planus.
Oral Surg. Oral Med. Oral Pathol. 69:585-590 (1990).
Holmstrup, P., J. J. Thorn, J. Rindum and J. J. Pindborg:
Malignant Development of Lichen Planus—Affected Oral
Mucosa. /. Oral Pathol. 17:219-225 (1988).
Horch, H., K. L. Gerlach and H. E. Schaefer: CO2 Laser
Surgery of Oral Premalignant Lesions. Oral Maxillofac.
Surg. 15:19-24(1986).
Jansen, C. T., R. Lehtinen, R. R. Happonen, A. Lehtinen and
K. Soderlund: Mouth PUVA: New Treatment for Recalcitrant Oral Lichen Planus. Photodermatology. 4:165166 (1987).
Jolly, M. and S. Nobile: Vitamin Status of Patients with Oral
Lichen Planus. Aust. Dent. J. 22:446-450 (1977).
Katz, J., J. Goultschin, R. Benoliel, I. Rotstein and S. Pisanti:
Lichen Planus Evoked by Periodontal Surgery. /. Clin.
Periodontol. 15:263-265 (1988).
Krogh, P., P. Holmstrup, J. J. Thorn, P. Vedtofte and J. J.
Pindborg: Yeast Species and Biotypes Associated with
Leukoplakia and Lichen Planus. Oral Surg. Oral Med.
Oral Pathol. 63:48-54 (1987).
Kutcher, A. H., E. V. Zegarelli, J. Hauptman and J. M.
Ragosta: Lack of Toxicity or Side Reactions Accompanying Topical Kenalog Therapy of Oral Lesions. Oral
Surg. Oral Med. Oral Pathol. 22:27-31 (1966).
Lacy, M. F., P. C. Reade and K. D. Hay: Lichen Planus: A
Theory of Pathogenesis. Oral Surg. Oral Med. Oral
Pathol. 54:521-525 (1983).
Lehtinen, R., R. P. Happonen, A. Kusilehto and C. Jansen:
A Clinical Trial of PUVA Treatment in Oral Lichen
Planus. Proc. Finn. Dent. Soc. 85:229-233 (1989).
Lehner, T. and C. Lyne: Adrenal Function During Topical
Oral Corticosteroid Treatment. Br. Med. J. 4:138-141
(1969).
Leopard, P. J. and D. E. Poswillo: Practical Cryosurgery for
Oral Lesions. Br. Dent. J. 136:185-196 (1974).
Levell, N. J., R. I. Macleod and J. M. Marks: Lack of Effect
of Cyclosporin Mouthwash in Oral Lichen Planus. Lancet. 337:796-797 (1991).
Lind, P.: Oral Lichenoid Reactions Related to Composite
Restorations. Acta Odontol. Scand. 46:63-65 (1988).
Loitz, G. A. and J. P. O'Leary: Erosive Lichen Planus of the
Tongue Treated With Cryosurgery. /. Oral Maxillofac.
Surg. 44:580-582 (1986).
Lozada, F. and J. Silverman: Topically Applied Fluocinonide
in an Adhesive Base in the Treatment of Oral
Vesiculoerosive Diseases. Arch. Dermatol. 116:190-201
(1980).
Lozada, F.: Prednisone and Azathioprine in the Treatment of
Patients with Vesiculoerosive Oral Diseases. Oral Surg.
Oral Med. Oral Pathol. 52:257-260 (1981).
Lozada, F., J. Silverman and C. Migliorati: Adverse Side
Effects Associated with Prednisone in the Treatment of
Patients With Oral Inflammatory Ulcerative Diseases. /.
Am. Dent. Assoc. 1091:269-270 (1984).
156
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Lozada-Nur, F. and M. Z. Huang: Open Preliminary Clinical
Trial of Clobetasol Propionate Ointment in Adhesive Paste
for Treatment of Chronic Oral Vesiculoerosive Diseases.
Oral Surg. Oral Med. Oral Pathol. 71:283-287 (1991).
Lowenthal, V. and S. Pisanti: Oral Lichen Planus According
to the Modern Medical Model. /. Oral Med. 39:22^226
(1984).
Lundstrom, I. M. C , G. B. Anneroth and K. Holmberg:
Candida in Patients with Oral Lichen Planus. Int. J. Oral
Surg. 13:226-238 (1984).
Malurstrom, M. and H. Leikomaa: Experiences with
Cryosurgery in the Treatment of Oral Lesions. Proc.
Finn. Dent. Soc. 76:117-123 (1980).
Massa, M. C. and R. S. Rogers, III: Griseofulvin Therapy of
Lichen Planus. Acta Derm. Venereol. 61:547-550 (1981).
Mathews, R. W., R. C. Pinkney and C. Scully: The Management of Desquamative Gingivitis with Dapsone. Ann.
Dent. 48:41-43 (1989).
McCarthy, F. P., P. L. McCarthy and G. Sklar: Chronic
Desquamative Gingivitis: A Reconsideration. Oral Surg.
Oral Med. Oral Pathol. 13:1300-1313 (1960).
Moschella, S. L.: Chemotherapy Used in Dermatology. Cutis. 19:603-612 (1977).
Murti, P. R., D. K. Daftary, R. B. Bhonsle, P. C. Gupta, F. S.
Mehta and J. J. Pindborg: Malignant Potential of Oral
Lichen Planus: Observation in 722 Patients from India. J.
Oral Pathol. 15:71-77 (1986).
Naylor, G. D.: Treating Erosive Lichen Planus with
Griseofulvin: A Report of Four Cases. Quintessence Int.
21:943-947 (1990).
Neumann-Jensen, B., P. Holmstrup and J. J. Pindborg: Smoking Habits of 611 Patients with Oral Lichen Planus. Oral
Surg. Oral Med. Oral Pathol. 43:410-413 (1977).
Peck, G. L.: Retinoids: Therapeutic Use in Dermatology.
Drugs. 24:341-351 (1982).
Pennys, N., A. Ackerman and N. Gottlieb: Gold Dermatitis.
Arch. Dermatol, 109:372-380 (1974).
Pigatto, P. D., G. Chiappino, A. Bigardi, N. Mozzanica and
A. F. Finzi: Cyclosporin A for Treatment of Severe Lichen Pianus. Br. J. Dermatol. 122:121-123 (1990).
Pindborg, J. J., F. S. Mehta, D. K. Daftary, R. C. Gupta and
R. B. Bhonsle: Prevalence of Oral Lichen Planus Among
7639 Indian Villagers in Kerala, South India. Acta Derm.
Venereol. 52:216-218 (1972).
Plemons. J. M., T. D. Rees and N. Y. Zachariah: Absorption
of Topical Steroid and Evaluation of Adrenal Suppression in Patients with Erosive Lichen Planus. Oral Surg.
Oral Med. Oral Pathol. 69:688-693 (1990).
Potts. A. J. C J. Hamburger and C. Scully: The Medication
of Patients with Oral Lichen Planus and Association of
Nonsteroidal Anti-Inflammatory Drugs with Erosive
Lesions. Oral Surg. Oral Med. Oral Pathol. 64:541-543
(1987).
Randall. J. and L. Cohen: Erosive Lichen Planus. Management of Oral Lesions with Intralesional Corticosteroid
Injections. /. Oral Med. 29:88-91 (1974).
Regezi. J. A., C. N. Ellis, J. C. V. Steward and T. A. Giustina:
Histologic Changes Associated with the Topical Use of
Isotretinoin on Oral Lichen Planus. Oral Surg. Oral Med.
Oral Pathol. 5:479-484 (1986).
Rogers, R. S., P. J. Sheridan and S. H. Nightingale:
Desquamative Gingivitis: Clinical, Histopathologic, Immunopathologic, and Therapeutic Observations. /. Am.
Acad. Dermatol. 7:729-735 (1982).
Ronbeck, B. A., P. O. Lind and P. S. Thrane: Desquamative
Gingivitis: Preliminary Observations with Tetracycline
Treatment. Oral Surg. Oral Med. Oral Pathol. 69:694697 (1990).
Rushton, R. J.: Treatment of Ulcerative Mouth Lesions with
Orabase. Br. J. Dermatol. 74:462-464 (1962).
Sato, M., H. Yoshida, T. Yanagawa, Y. Yura, M. Urata, T.
Nitta, M. Azuma and Y. Hayashi: Therapeutic Effect of
Human Fibroblast Interferon on Premalignant Lesions
Arising in the Oral Mucosa. A Pilot Study. Int. J. Oral
Surg. 14:184-194(1985).
Schuppli, R.: The Efficacy of a New Retinoid (RO 10-9359)
in Lichen Planus. Dermatologica. 157:60-63 (1978).
Scully, C. and M. El-Kom: Lichen Planus: Review and
Update on Pathogenesis. J. Oral Pathol. 14:431-458
(1985).
Sehgal, V. N., G. J. S. Abraham and G. B. Malik: Griseofulvin
Therapy in Lichen Planus. A Double-Blind Control Trial.
Br. J. Derm. 87:383-385 (1972).
Silverman, S., Jr., F. Lozada-Nur and C. Migliorati: Clinical
Efficacy of Prednisone in the Treatment of Patients with
Oral Inflammatory Ulcerative Diseases: A Study of 55
Patients. Oral Surg. Oral Med. Oral Pathol. 59:360-363
(1985).
Silverman, S., Jr., M. Gorsky and F. Lozada-Nur: A Prospective Follow-up Study of 570 Patients with Oral Lichen
Planus; Persistence, Remissions, and Malignant Association. Oral Surg. Oral Med. Oral Pathol. 60:30-34 (1985).
Silverman, S., Jr., M. Gorsky, F. Lozada-Nur and K. Giannotti:
A Prospective Study of Findings and Management in 214
Patients With Oral Lichen Planus. Oral Surg. Oral Med.
Oral Pathol. 72:665-670 (1991).
Simon, M., Jr. and O. P. Hornstein: Prevalence Rate of
Candida in the Oral Cavity of Patients with Oral Lichen
Planus. Arch. Dermatol. Res. 267:317-318 (1980).
Sleeper, H. R.: Intralesional and Sublesional Injection of
Triamcinolone Acetonide for Oral Lichen Planus. Yale J.
Biol. Med. 40:164-165 (1967).
Sloberg, K., K. Hersle, H. Mobacken and H. Thilander:
Topical Tretinoin Therapy in Oral Lichen Planus. Arch.
Dermatol. 115:716-718 (1979).
Sloberg, K., K. Hersle, H. Mobacken and H. Thilander:
Severe Oral Lichen Planus: Remission and Maintenance
With Vitamin A Analogues. /. Oral Pathol. 12:413-411
(1983).
Staus, M. E. and W. F. Bergfeld: Treatment of Oral Lichen
Planus with Low-Dose Isotretinoin. J. Am. Acad.
Dermatol. 11:527-528 (1984).
Stuttgen, G.: Oral Vitamin A Acid Therapy. Acta Derm.
Venereol. 55:174-175 (1975).
Takeuchi, Y., I. Tohnai, T. Kaneda and H. Nagura: Immunohistochemical Analysis of Cells in Mucosal Lesions of
Oral Lichen Planus. J. Oral Pathol. 17:367-373 (1988).
Tal, H. and B. Rafkin: Cryosurgical Treatment of a Gingival
Lichen Planus: Report of Case. J. Am. Dent. Assoc.
113:629-631 (1986).
157
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.
Thorn, J. J., P. Holmstrup, J. Rindum and J. J. Pindborg:
Course of Various Clinical Forms of Oral Lichen Planus.
A Perspective Follow-Up Study of 611 Patients. / . Oral
Pathol. 17:213-218 (1988).
Tyldesley, W. R. and S. M. Harding: Betamethasone Valerate Aerosol in the Treatment of Oral Lichen Planus. Br.
J. Dermatol. 96:659-662 (1977).
Van Dijk, T. S. H. and J. L. Van Velde: Treatment of
Pemphigus and Pemphigoid with Azathioprine.
Dermatologica. 147:179-185 (1973).
Vedtofte, P., P. Holmstrup, E. H. Hansen and J. J. Pindborg:
Surgical Treatment of Premalignant Lesions of the Oral
Mucosa. Int. J. Oral Maxillofac. Surg. 16:656-664 (1987).
Vincent, S. D., P. G. Fotos, K. A. Baker and T. P. Williams:
Oral Lichen Planus: The Clinical, Historical, and Therapeutic Features of 100 Cases. Oral Surg. Oral Med. Oral
Pathol. 70:165-171 (1990).
Wagner, H.: Cyclosporin A: Mechanism of Action. Transplant Proc. 15:523-536 (1983).
Welton, W.: How I Treat Lichen Planus. Postgrad. Med.
46:196-197 (1969).
Whitten, J. B.: Intraoral Lichen Planus Simplex: An
Ultrastructure Study. / . Periodont. 41:261-264
(1970).
Wilson, E.: On Leichen Planus. /. Cutan. Med. Dis. Skin.
3:117-132(1869).
Woo, T. Y.: Systemic Isotretinoin Treatment of Oral and
Cutaneous Lichen Planus. Cutis. 35:385-393 (1985).
Zegarelli, D. J.: Multimodality Steroid Therapy of Erosive
and Ulcerative Oral Lichen Planus. / . Oral Med. 38:127130 (1983).
Zegarelli, D. J.: Ulcerative and Erosive Lichen Planus.
Treated by Modified Topical Steroid and Injection Steroid Therapy. NYS Dent. J. 53:23-24
(1987).
Zegarelli, D. J., A. Kutscher and A. Mehrhof: Long-standing
Lozenges with Triamcinolone Acetonide. NYSJ Med.
69:2463-2646 (1969).
158
Downloaded from cro.sagepub.com by guest on September 9, 2014 For personal use only. No other uses without permission.