* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mucomist ® DT Prescribing information
Survey
Document related concepts
Transcript
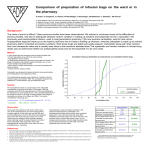
Mucomist® Acetylcysteine Respirator Solution, Dispersible Tablet Description Acetylcysteine is a derivative of the amino acid, cysteine. Mucomist® DT contains Acetylcysteine 600 mg. Mucomist® DT (dispersible tablet) is a new pharmaceutical form that ensures optimum stability, rapid dissolution, maximal absorption, high bioavailability in a perfectly calibrated dosage. Mucomist® Respirator Solution contains 20% or 200 mg/ml Acetylcysteine and available as sterile, unpreserved solution (not for injection). The 20% inhalation solution is given by nebulization or by instillation intratracheally. Pharmacology Acetylcysteine has a mucolytic activity through its free sulfhydryl group. It acts directly on the mucoproteins to open the disulfide bonds and thus lowers the viscosity of the mucous and facilitates its removal by the muco-cillary action and expectoration. Acetylcysteine improves the phagocytic capacity of the alveolar macrophages, thus protecting lungs from a variety of insults. Acetylcysteine is a precursor to glutathione, the most important intra & extra-cellular antioxidant (the safest & most convenient). Indication and Use As Mucolytic in pulmonary disease: Respiratory tract disorders associated with excessive viscous mucous such as- chronic bronchopulmonary disease (chronic emphysema, emphysema with bronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis and primary amyloidosis of the lung), acute bronchopulmonary disease (pneumonia, bronchitis, tracheobronchitis), pulmonary complications of cystic fibrosis, tracheostomy care, pulmonary complications associated with surgery; post-traumatic chest conditions, atelectasis due to mucous obstruction. As an antidote for Paracetamol overdose: Acetylcysteine, administered orally, is indicated as an antidote to prevent or lessen hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of Paracetamol. As for diagnostic bronchial studies: Such as bronchograms, bronchospirometry, and bronchial wedge catheterization. Dosage and Administration Mucomist ® DT: The dispersible tablet should be dissolved in 1/2 glass of water before use (preferably in the evening). The duration of treatment should be 5 to 10 days in the acute phase. It may be continued in the chronic state for up to 6 months or according to the advice of the physician. As a mucolytic: Adults: 600 mg daily as a single dose. In Paracetamol overdose: Initially 140 mg/kg, followed by 70 mg/kg every 4 hours for an additional 17 doses. As an antidote, Acetylcysteine is reported to be very effective when administered within 8 hours of Paracetamol overdose, with the protective effect diminishing after this time. Initiation of treatment after a lapse of 15 hours has previously been thought to be ineffective, but recent studies suggest that beneficial results may still be obtained. Mucomist ® Respirator Solution: The 20% solution may be diluted to a lesser concentration with either Sodium Chloride for injection, Sodium Chloride for inhalation, sterile water for injection, or sterile water for inhalation. As a mucolytic: Adult: 5-10 ml of 10% or 20% solution by nebulizer every 6-8 hr PRN. Children: 1-11 months: 1-2 ml of 20% or 2-4 ml of 10% solution by nebulizer every 6-8 hr PRN. 12 months-11 years: 3-5 ml of 20% or 6-10 ml of 10% solution by nebulizer every 6-8 hr PRN. Below 12 years: 5-10 ml of 10/20% solution by nebulizer every 6-8 hr PRN. Diagnostic Bronchograms: 1-2 ml of 20% or 2-4 ml of 10% solution 2-3 times by nebulization or by instillation intratracheally prior to procedure. Nebulization tent or croupette: This form of administration requires very large volumes of the solution, occasionally as much as 300 ml during a single treatment period. If a tent or croupette must be used, the recommended dose is the volume of acetylcysteine (using 20%) that will maintain a very heavy mist in the tent or croupette for the desired period. Administration for intermittent or continuous prolonged periods, including overnight, may be desirable. Direct Instillation: When used by direct instillation, 1-2 ml of a 20% solution may be given as often as every hour. When used for the routine nursing care of patients with tracheostomy, 1-2 ml of a 20% solution may be given every 1-4 hours by instillation into the tracheostomy. Pregnancy Pregnancy Category- B (US FDA). Lactation It is not known if this drug is excreted in human milk. Side Effects Generally, Acetylcysteine is well tolerated. However, mild effects such as nausea, headache, tinnitus, urticaria, stomatitis, rhinorrhoea, chills, fever, bronchospasm may be observed. Occasional cases of nausea and dyspepsia, rare cases of urticaria may be observed. Contraindications Acetylcysteine is contraindicated in those patients who are sensitive to it. Precaution Acetylcysteine should be given in caution in asthma patients. Drug interaction After taking Acetylcysteine orally it increases the bioavailability of amoxicillin, but shows no effect on doxycycline and reduces the absorption of cefalexine. Acetylcysteine seems to increase the effects of nitroglycerin. Taking Acetylcysteine at the same time as activated charcoal might decrease how well it works for preventing poisoning. Pharmaceutical Precaution Mucomist ® Respirator Solution: The physical and chemical compatibility of acetylcysteine solutions with certain other drugs that might be concomitantly administered by nebulization, direct instillation, or topical application has been studied. Acetylcysteine should not be mixed with certain antibiotics. For example, antibiotics like tetracycline hydrochloride, oxytetracycline hydrochloride, and erythromycin lactobionate were found to be incompatible when mixed in the same solution. These agents may be administered from separate solutions if administration of these agents is desirable. If it is deemed advisable to prepare an admixture, it should be administered as soon as possible after preparation. Do not store unused mixtures. Storage and Handling Instructions Mucomist ® Respirator Solution: Do not contain an antimicrobial agent; hence, care must be taken to minimize contamination of the sterile solution. Store below 25o C, away from light. Keep out of reach of children. Mucomist ® DT: Keep in a cool and dry place. Protect from light. Keep out of the reach of children. Commercial Pack Mucomist® DT: Box containing 30 dispersible tablets in 3x10’s Aluminium strips. Each dispersible tablet contains Acetylcysteine USP 600 mg. Mucomist® Respirator Solution: Box containing 1 x 5 ampoules of 3 ml in blister pack. Each 3 ml ampoule contains Acetylcysteine USP 600 mg i.e. 200 mg/ml or 20% solution. Manufactured by BEXIMCO PHARMACEUTICALS LTD. TONGI, BANGLADESH IL 9450 120214 ® Mucomist is a registered trademark of Beximco Pharmaceuticals Ltd.