* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NItric Oxide and Prostaglandins: Mediators of Pathogenesis in
Population genetics wikipedia , lookup
Behavioural genetics wikipedia , lookup
Heritability of IQ wikipedia , lookup
Genome evolution wikipedia , lookup
Genomic imprinting wikipedia , lookup
Ridge (biology) wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene expression profiling wikipedia , lookup
Minimal genome wikipedia , lookup
History of genetic engineering wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Microevolution wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Human genetic variation wikipedia , lookup
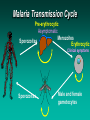
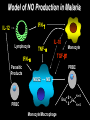
Molecular Epidemiology and Susceptibility to Malaria Infection Douglas Jay Perkins, Ph.D. University of Pittsburgh Graduate School of Public Health Department of Infectious Diseases and Microbiology Centers for Disease Control and Prevention Division of Parasitic Diseases-Immunology Branch Molecular Vaccine Section, Atlanta, GA Malaria Transmission Cycle Pre-erythrocytic Asymptomatic Merozoites Sporozoites Erythrocytic Clinical symptoms Sporozoites Male and female gametocytes Malaria in Humans • Four species of genus Plasmodium infect humans: P. falciparum, P. vivax, P. ovale, and P. malariae • Transmitted by female Anopheline mosquito • 300-500 million clinical cases per year Populations at Risk • Infants, young children, and pregnant women in malaria endemic regions – Greater than 3 million deaths (primarily in children less than 5 y/o due to non-immune status) • Non-immune individuals traveling through and/or living in malaria endemic regions – 35 million non-immune individuals travel through malaria endemic regions every year Clinical Features of P. falciparum • P. falciparum can cause severe malaria: -hyperparasitemia -severe anemia -hypoglycemia -respiratory distress -cerebral malaria • Molecular determinants that regulate mild versus severe disease largely unknown Current Situation: Major International Health Problem • Rapidly expanding number of clinical cases each year • Growing problem of antimalarial drug resistance with few novel therapeutics available • Lack of an effective vaccine Potential Solutions • Gain an understand of the genetic and immunologic basis of protective immunity • Identify novel targets for therapeutic intervention • Determine reliable markers for measuring protection and pathogenesis for use in pharmacologic and/or vaccine trials Genetic Susceptibility to Malaria • At least 10,000 years of “pressure” on the human genome from the malaria parasite • In 1948 J.B.S. Haldane suggested that the high frequency of thalassemia in Mediterranean populations might confer a heterozygote advantage against malaria • Thalassemias are defects in synthesis of either a- or bglobin chains of hemoglobin (hemoglobin adult = a2b2) • Mechanism of protection may be related to increased binding of antibodies and/or increased retention of fetal hemoglobin Sickle Cell Gene and Resistance to Malaria • Over 400 abnormal hemoglobins but only three reach polymorphic frequencies (S, C, & E) • Homozygous state (SS) = sickle cell disease • Heterozygous state (SC) = protection from malaria • Mechanism unknown but red blood cells from (SC) individuals have reduced parasite growth and impaired invasion under low O2 tension • In addition to red cell abnormalities, there are many other genetic changes…….. Host Response Genes and Susceptibility to Malaria • In 1993 Murphy compared sequences of human and rodent genes and found greater variability among host defense genes • Polymorphisms in cytokines genes (e.g. TNF-a) and effector molecules (e.g. nitric oxide, NO) are now being investigated • Study of genetic variation may utilize several types of DNA markers to analyze candidate susceptibility genes Single base pair variations = SNPs Microsatellite or variable number tandem repeats (VNTRs) Overview Part 1. NOS2 (G –954C) in Gabonese Children with Severe Malarial Anemia Part 2. NOS2 (G –954C) in Tanzanian Children with Cerebral Malaria Part 3. NOS2 (G –954C) in Kenyan Children with Severe Malarial Anemia Nitric Oxide Biosynthesis L-Arginine NOS L-Citrulline + NO L-NMMA Aminoguanidine NO2- NO3- NOS Enzyme Assay Cellular Lysate [14C]L-Arg remains [14C]L-Arg Co-factors [14C]L-Cit [14C]L-Cit flows through Cation Exchange Column Nitric Oxide Synthase eNOS & nNOS iNOS NOS3 NOS2 NOS1 Constitutive Expression Inducible Expression - Ca2+- and CalmodulinDependent - Ca2+- and Calmodulin- NO Synthesis for Normal Physiologic Function NO Synthesis in the Setting of Inflammation Independent Nitric Oxide: Previous Observations in Malaria • Nitric oxide production is anti-plasmodial in vitro and in vivo -(Oswald et al.,Comp Biochem Physiol Pharmacol Toxicol Endocrino, 1994; 108:11-18) • Elevated NO metabolites are associated with accelerated clinical cure and increased parasitologic clearance in Gabonese adults and children -(Kremsner et al., Trans R Soc Trop Med Hyg, 1996; 90: 44-47) NO appears protective against malaria Model of NO Production in Malaria IFN-g IL-12 Lymphocyte IL-10 Monocyte TNF-a TGF-b1 IFN-a Parasitic Products PRBC NOS2 NO S Enz Fe S PRBC Monocyte/Macrophage N=O N=O Hypothesis Increased capacity of the host to generate nitric oxide is protective against severe malaria