* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download EXPERIMENT Q-5 Electron Diffraction Abstract References Pre-Lab
Survey
Document related concepts
Scanning tunneling spectroscopy wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Diffraction grating wikipedia , lookup
Diffraction topography wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Photomultiplier wikipedia , lookup
Transmission electron microscopy wikipedia , lookup
Gaseous detection device wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Diffraction wikipedia , lookup
Transcript
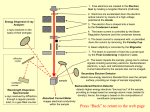
EXPERIMENT Q-5 Electron Diffraction Abstract The wave nature of the electron is examined by directing an accelerated beam of electrons at a polycrystalline sample. The resulting electron diffraction pattern is studied as a function of the accelerating voltage, and the data is used to determine Planck's constant, h. References Taylor, Zafiratos & Dubson, Modern Physics, 2004, Section 6.3 and Figs 4.7 & 4.10 for x-rays Reese, University Physics, 2000, Section 26.15 Pre-Lab Please do this section before coming to lab. By now you've seen an experiment in which light behaves like particles instead of waves. In this lab, electrons behave like waves instead of particles. A beam of electrons is "diffracted" by a crystalline sample according to the "Bragg law", an equation originally used to describe X-ray diffraction. The beam of electrons with common speed v is produced by an "electron gun". The electron gun consists of two parallel metal plates, the cathode and the anode, connected to a power supply that makes the anode voltage V volts more positive than the cathode voltage. A filament heats the cathode, ejecting electrons by thermionic emission. The electrons have negligible speed to begin with, but are attracted toward the positive anode, speeding up along the way. When they reach the anode their kinetic energy is equal to the amount of electrical potential energy that was lost, i.e. KE = eV In this equation, e is the electron charge, and the absolute value signs are used to guarantee that KE comes out positive. Using the "regular" equation for KE, solve the equation to get the speed of electrons reaching the anode. Some of these electrons pass through a small hole in the anode, thereby emerging from the electron gun with this speed, v. Look up the Bragg law of diffraction in the references. Although it looks like other optics equations you know, there are differences in what the symbols mean. Note that the angle θ is measured from the incident beam to the surface of the crystal, not to the "normal" as in Snell's law and the law of reflection. Note also that d is a "depth", i.e. the separation between crystal planes as you move downward from the crystal's surface. It's not like the distance between slits in the equation for 2-slit interference. The new thing in this experiment is that the electrons have a wavelength, λ, that is inversely proportional to their momentum. This relationship is known as the de Broglie relation, and is the starting point for "quantum mechanics". Look up the de Broglie equation in the references, and write down the value for the proportionality constant (h, known as "Planck's constant") in Joule-seconds and in eV-seconds. Starting from the de Broglie relationship, satisfy yourself that units of energy-time are the correct units for h. Read the Procedure section below, to help prepare yourself for lab. Think about QUESTIONS 1 and 2, so you can quickly answer when you come to them. As always, feel free to ask questions! 82 Q-5 Apparatus Teltron electron diffraction tube Teltron 0-5000 V power supply Dial Caliper Calipers Bar magnet Meter stick Procedure Connect the filament leads from the electron tube to the 6.3Volt terminals on the power supply, asking an instructor for assistance in identifying the various leads. Connect the red and black accelerating voltage leads to the "+" and "-" high voltage power supply terminals, respectively. WARNING!! THERE WILL BE UP TO 6000V ON THIS RED WIRE. THIS CAN BE FATAL, SO DON'T TOUCH IT!! Be sure the voltage is turned all the way down. Reduce the room lights, turn on the supply, and slowly increase the accelerating voltage until the electron beam produces a small spot near the center of the fluorescent screen. Convince yourself that the beam producing this spot is an electron beam by holding a bar magnet in different orientations near the diffraction tube. QUESTION 1: Why does the bar magnet prove the spot is made by electrons and not light or protons? (Hint: think of the Lorentz Force Law) Increase the accelerating voltage to about 3000 volts and look for evidence of electron diffraction. You should see a pair of circular rings concentric with the central spot. QUESTION 2: The Bragg law shows that diffracted electrons from a single crystal emerge at only one diffraction angle. This experiment uses a polycrystalline, or "powder" sample. Why do the diffracted electrons form a circle on the screen instead of a single off-axis point? Record the diameter, 2r, of both these rings as a function of accelerating voltage, obtaining at least 10 different values of each ring diameter in the voltage range 2500-5000 volts. Make certain you discuss the method used to determine the diameters and their uncertainty. Referring to Figure 1, carefully measure the distance, L, from the sample to the screen center, justifying its uncertainty. Finally, measure the diameter, 2R, of the spherical portion of the diffraction tube, including uncertainty. Figure 1: Electron Diffraction Tube Q-5 83 Sample Calculation Using the geometry of Figure 1, determine the exact solution for φ (hint: it is NOT tanφ = r / L). Make a drawing showing Bragg diffraction from a crystal. On this drawing, show the angles φ and θ, and derive the relationship between the two. From these geometrical considerations and your data, calculate the best value of the largest diffraction angle, θ, that was produced in your experiment. Dismantle the apparatus, unplug any equipment, and return the lab to its original state. Analysis For each accelerating voltage, two diffraction rings were observed. Make separate Excel tables for the inner and outer rings, showing your data for ring diameter as a function of voltage. For each trial, compute the diffraction angle, θ, using a formula based on the sample calculation. Assuming that at a given accelerating voltage the electron beam has a single wavelength, the two rings must be due either to: a) different orders of diffraction from a single set of lattice planes, or b) two different sets of lattice planes with different lattice spacings, d. In a separate column of your table for the outer ring, determine which of these possibilities applies in your experiment by calculating the ratio sinθ θo/sinθ θi - where θi and θo are the diffraction angles for the inner and outer rings. QUESTION 3: What should the ratio (sinθo)/(sinθi) be if the two rings correspond to first and second order diffraction from a single set of lattice planes? It is known that the inner ring corresponding to each voltage results from diffraction from a set of lattice planes with di = 2.1 Ǻ (i.e. 2.1 x 10-10 m). In another column of your table for the outer ring, use this di, the ratio of the sines, and The Bragg Law to determine do, the spacing of the lattice planes responsible for the outer diffraction ring. Find an average and standard deviation of this lattice spacing and state a final result. In your worksheet table for the inner ring, use the Bragg law, the given lattice spacing, and the deBroglie relation to find the electron wavelengths, λ, λ the electron momentum, p, and the best result for Planck's constant, h, corresponding to each accelerating voltage. Find an average and standard deviation for h, and state a final result. Write a conclusion that summarizes and interprets your results. Suggest ways you could improve the results if you were to repeat the experiment, mention problems you had in lab, etc... 84 Q-5