* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download US Preventive Services Task Force
Epidemiology of metabolic syndrome wikipedia , lookup
Maternal physiological changes in pregnancy wikipedia , lookup
HIV and pregnancy wikipedia , lookup
Public health genomics wikipedia , lookup
Infection control wikipedia , lookup
Preventive healthcare wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Newborn screening wikipedia , lookup
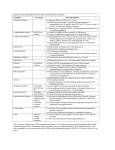
US Preventive Services Task Force Recommendations for Children
March, 2014
Recommended
(A or B Grade)
Preventive Service
Screening
Body Measurement
Blood Pressure1
Height/Weight/BMI2
Hip Dysplasia (infant exam or US)3
Idiopathic Scoliosis (adolescent exam)4
Metabolic
Lipids5
Lead6
Anemia (infants)7
Hyperbilirubinemia (infants)8
Newborn (PKU, thyroid, hemoglobinopathy)9
Cancer
Testicular (exam, self-exam)10
Sensory
Hearing11
Vision (strabismus, amblyobia, acuity)12
Speech and Language13
Recommendation Key
Insufficient Evidence/No recommendationi
(I or C Grade)
Recommend Against
(D Grade)
General Population
High Risk Population
Insufficient evidence
>6 years
Obesity—insufficient evidence
Insufficient evidence
Recommend against
No specific recommendation
No specific recommendation
No specific recommendation
No specific recommendation
No specific recommendation
Insufficient evidence
Recommend against
Insufficient evidence
Insufficient evidence
At birth
No specific recommendation
Insufficient evidence
Premature or low birth weight infants
No specific recommendation
At birth
Recommend against
No specific recommendation
Newborn
> 3 years—recommend against
3-5 years at least once
<3 years—insufficient evidence
Preschool—insufficient evidence
Newborn
Insufficient evidence
No specific recommendation
No specific recommendation
No specific recommendation
Preventive Service
Infectious Diseases
Tuberculosis (PPD)14
Chlamydia15
Hepatitis C16
HIV17
Syphilis (serology)18
Gonorrhea (culture or nucleic acid tests)19
Mental Health
Depression20
Suicide21
General Population
High Risk Population
Insufficient evidence
Medically underserved, low income, foreign born
(Asia, Africa, Latin America), alcoholic, IVDA,
residents of institutions, close contact with TB, and
HIV, diabetes, renal failure. ? periodicity
F, sexually active or pregnant, ? periodicity
M, insufficient evidence
Insufficient evidence
High risk sexual activity or IV drug . F, pregnant
High risk sexual activity. F, pregnant
F, sexually active, ? periodicity
M, insufficient evidence
F, no recommendation
M, insufficient evidence
Recommend against
No recommendation
Recommend against
Recommend against
Recommended for adolescents
Insufficient evidence
No specific recommendation
No specific recommendation
No recommendation
Insufficient evidence
F, childbearing age
Newborns
6 mo. to 5 years if low fluoride exposure
6-12 mo. premature, low birth weight
No specific recommendation
No specific recommendation
Alcohol Misuse27
School age and adolescents—prevention counseling
and education.
Insufficient evidence
Healthy Diet28
Physical Activity29
Obesity30
Skin Cancer Prevention31
Dental Caries32
Sexually Transmitted Infections (STIs)33
Child Maltreatment34
Insufficient evidence
Insufficient evidence
Insufficient evidence
Insufficient evidence
Insufficient evidence
Insufficient evidence
Insufficient evidence
F, pregnant smokers—pregnancy-tailored
counseling
F, pregnant—inform of harmful effects of alcohol
on fetus
Insufficient evidence
Insufficient evidence
Insufficient evidence
Fair complexion
Insufficient evidence
Adolescents at increased risk for STIs
No specific recommendation
Chemoprevention
Fluoride Supplement22
Iron Supplement23
Folic Acid24
Ophthalmia Neonatorum (antibiotics)25
Counseling
Tobacco Use26
Rationale
1
Blood Pressure. The USPSTF found poor evidence that routine blood pressure measurement accurately identifies children and adolescents at increased risk for
cardiovascular disease, and poor evidence to determine whether treatment of elevated blood pressure in children or adolescents decreases the incidence of
cardiovascular disease. As a result, the USPSTF could not determine the balance of benefits and harms of routine screening for high blood pressure in children
and adolescents. (2003)
2
Height/Weight/BMI. Previously, the USPSTF found adequate evidence that BMI was an acceptable measure for identifying children and adolescents with
excess weight. The USPSTF found adequate evidence that multicomponent, moderate- to high-intensity behavioral interventions for obese children and
adolescents aged 6 years and older can effectively yield short-term (up to 12 months) improvements in weight status. Inadequate evidence was found regarding
the effectiveness of low-intensity interventions. There is adequate evidence that the harms of behavioral interventions are no greater than small. The USPSTF
concludes that there is moderate certainty that the net benefit is moderate for screening for obesity in children aged 6 years and older and for offering or referring
children to moderate- to high-intensity interventions to improve weight status. This recommendation applies to children and adolescents aged 6 to 18 years. The
USPSTF is using the following terms to define categories of increased BMI: overweight is defined as an age- and gender-specific BMI between the 85th and 95th
percentiles, and obesity is defined as an age- and gender-specific BMI at ≥95th percentile. The USPSTF did not find sufficient evidence for screening children
younger than 6 years. In 2005, the USPSTF found adequate evidence that BMI was an acceptable measure for identifying children and adolescents with excess
weight. BMI is calculated from the measured weight and height of an individual. The USPSTF found that effective comprehensive weight-management
programs incorporated counseling and other interventions that targeted diet and physical activity. Interventions also included behavioral management techniques
to assist in behavior change. Interventions that focused on younger children incorporated parental involvement as a component. Moderate- to high-intensity
programs involved >25 hours of contact with the child and/or the family over a 6-month period and showed results including improved weight status, defined as
an absolute and/or relative decrease in the BMI 12 months after the beginning of the intervention. Most participants were obese, and it is not known whether
these results can be applied to children who are overweight but not obese. In addition, evidence was limited on the long-term sustainability of BMI changes
achieved through behavioral interventions and on the trajectory of weight gain in children and adolescents. Interventions generally took place in referral settings,
and the results can only be generalized to children who follow through on treatment. Low-intensity interventions, defined as ≤25 contact hours over a 6-month
period, did not result in significant improvement in weight status. Interventions that combined pharmacologic agents (sibutramine or orlistat) with behavioral
interventions resulted in modest short-term improvement in weight status in children aged 12 years and older. There were no long-term data on the maintenance
of improvement after discontinuation of medications. The magnitude of the harms of these drugs in children could not be estimated with certainty. Adverse
effects included elevated heart rate, elevated blood pressure, and adverse gastrointestinal effects. Sibutramine, a centrally acting appetite suppressant, has been
approved by the US Food and Drug Administration (FDA) for use in adolescents aged 16 years and older. Orlistat, a lipase inhibitor, has been approved by the
FDA for use in adolescents aged 12 years and older. Neither sibutramine nor orlistat has been approved for use in pediatric populations younger than 12 years.
(2010)
3
Hip Dysplasia. The pathophysiology and natural history of developmental dysplasia of the hip (DDH) are poorly understood. There is evidence that screening
leads to earlier identification; however, 60% to 80% of the hips of newborns identified as abnormal or as suspicious for DDH by physical examination and >90%
of those identified by ultrasound in the newborn period resolve spontaneously, requiring no intervention. There is poor evidence (poor quality studies) of the
effectiveness of both surgical and non-surgical interventions; avascular necrosis of the hip (AVN) is reported in 0% to 60% of children who are treated for DDH.
Thus, the USPSTF was unable to assess the balance of benefits and harms of screening for DDH but was concerned about the potential harms associated with
treatment of infants identified by routine screening. (2006)
4
Idiopathic Scoliosis. The USPSTF did not find good evidence that screening asymptomatic adolescents detects idiopathic scoliosis at an earlier stage than detection
without screening. The accuracy of the most common screening test—the forward bending test with or without a scoliometer—in identifying adolescents with idiopathic
scoliosis is variable, and there is evidence of poor followup of adolescents with idiopathic scoliosis who are identified in community screening programs. The USPSTF
found fair evidence that treatment of idiopathic scoliosis during adolescence leads to health benefits (decreased pain and disability) in only a small proportion of people.
Most cases detected through screening will not progress to a clinically significant form of scoliosis. Scoliosis needing aggressive treatment, such as surgery, is likely to be
detected without screening. The USPSTF found fair evidence that treatment of adolescents with idiopathic scoliosis detected through screening leads to moderate harms,
including unnecessary brace wear and unnecessary referral for specialty care. As a result, the USPSTF concluded that the harms of screening adolescents for idiopathic
scoliosis exceed the potential benefits. (2004)
5
Lipids. There is insufficient evidence to recommend routine screening in children, adolescents, or young adults. For adolescents and young adults who have a family
history of very high cholesterol, premature CHD in a first-degree relative (before age 50 in men or age 60 in women), or major risk factors for CHD screening may be
recommended on other grounds: the greater absolute risk attributable to high cholesterol in such persons, and the potential long-term benefits of early lifestyle
interventions in young persons with high cholesterol. Recommendations against routine screening in children may be made on other grounds, including the costs and
inconvenience of screening and follow-up, greater potential for adverse effects of treatment, and the uncertain long-term benefits of small reductions in childhood
cholesterol levels. (1996)
6
Lead. Blood lead levels in children have declined dramatically in the United States over the past two decades. However, segments of the population remain at increased
risk for higher blood lead levels. Even relatively low blood lead levels are associated with neurotoxic effects in children. Severely elevated blood lead levels in
symptomatic pregnant women are associated with poor health outcomes; however, lead levels in this range are rare in the U.S. population. There is good evidence that
venous sampling accurately detects elevated blood lead levels and fair evidence that validated questionnaires are modestly useful in identifying children at increased risk
for elevated blood lead levels. The USPSTF found good quality evidence that interventions do not result in sustained decreases in blood lead levels and found insufficient
evidence (no studies) evaluating residential lead hazard control efforts (i.e., dust or paint removal, soil abatement, counseling, or education) or nutritional interventions
for improving neurodevelopmental outcomes in children with mild to moderately elevated blood lead levels. The USPSTF found no evidence examining the effectiveness
of screening or interventions in improving health outcomes in asymptomatic pregnant women. Given the low prevalence of elevated blood lead levels in children at
average risk and asymptomatic pregnant women, the magnitude of potential benefit cannot be greater than small. A theoretical benefit of screening is that identification
may prevent lead poisoning of other individuals in a shared environment, but the magnitude of this theoretical benefit is uncertain. There is good quality evidence that
chelation treatment in asymptomatic children does not improve neurodevelopmental outcomes and is associated with a slight diminution in cognitive performance.
Chelation therapy may result in transient renal, hepatic, and other toxicity, mild gastrointestinal symptoms, sensitivity reactions, and rare life-threatening reactions.
Residential lead-based paint and dust hazard control treatments may lead to acutely increased blood lead levels from improper removal techniques. Potential harms of
screening are false-positive results, anxiety, inconvenience, work or school absenteeism, and financial costs associated with repeated testing. Although the exact
magnitude of these known and potential harms is uncertain, the overall magnitude is at least small. No studies have directly addressed the harms of screening and
interventions for pregnant women. Although there is little specific evidence concerning the potential harms of interventions for pregnant women with elevated blood lead
levels, the magnitude of harms from such interventions is also at least small. The USPSTF concluded that the evidence is insufficient to assess the balance between
potential benefits and harms of routine screening for elevated blood lead levels in children at increased risk. Given the significant potential harms of treatment and
residential lead hazard abatement, and no evidence of treatment benefit, the USPSTF concluded that the harms of screening for elevated blood lead levels in children at
average risk and in asymptomatic pregnant women outweigh the benefits.(2006)
7
Anemia. Iron deficiency anemia is associated with psychomotor and cognitive abnormalities in children. The prevalence of iron-deficiency anemia has remained stable
over the last decade in the general U.S. population and continues to be greatest among minority and poor children. A validated risk assessment tool to guide primary care
physicians in identifying individuals who would benefit from iron supplementation has not been developed. The USPSTF found fair evidence that iron supplementation
(e.g., iron–fortified formula or iron supplements) may improve neurodevelopmental outcomes in children at increased risk for iron deficiency anemia. The USPSTF
found poor evidence (poor quality and conflicting studies) that iron–fortified formula or supplementation improves neurodevelopmental outcomes in children aged 6 to
12 months if they are not at increased risk for iron deficiency anemia. The USPSTF found fair evidence that oral iron supplementation increases the risk for unintentional
overdose and gastrointestinal symptoms. Given appropriate protection against overdose, these harms are small. The moderate benefits of iron supplementation in
asymptomatic children aged 6 to 12 months who are at increased risk for iron deficiency anemia outweigh the potential harms. The USPSTF was unable to determine the
balance between the benefits and harms of iron supplementation in children aged 6 to 12 months who are at average risk for iron deficiency anemia. (2006)
8
Hyperbilirubinemia. The exact incidence of chronic bilirubin encephalopathy is not known but is very low; in 1 study, 90 cases were documented in term and
near-term infants in 21 states over a period of 17 years. In a recent prospective study in the United Kingdom and Ireland, the incidence of chronic bilirubin
encephalopathy was estimated at 0.9 per 100 000 live births. Efforts have been made by clinicians to eliminate this rare but devastating condition by instituting
system-level measures to screen for hyperbilirubinemia and by aggressively managing high bilirubin levels. There is adequate evidence that screening using risk
factors and/or hour-specific bilirubin measurement can identify infants at risk of developing hyperbilirubinemia. However, not all children with chronic bilirubin
encephalopathy have a history of hyperbilirubinemia, and there is no known screening test that will reliably identify all infants who are at risk of developing
chronic bilirubin encephalopathy. Early treatment can decrease the number of infants with elevated serum bilirubin levels. However, the USPSTF found
inadequate evidence that treating elevated bilirubin levels in term or near-term infants to prevent severe hyperbilirubinemia resulted in the prevention of chronic
bilirubin encephalopathy. Hyperbilirubinemia is commonly treated with phototherapy, and severe hyperbilirubinemia may be treated with exchange blood
transfusion. The USPSTF found inadequate evidence regarding the harms of phototherapy. Potential harms of phototherapy include weight loss, gastrointestinal
problems, interruption of breastfeeding and disruption of the maternal-infant relationship, and possibly growth of melanocytic nevi. Significant morbidity (apnea,
bradycardia, cyanosis, vasospasm, thrombosis, necrotizing enterocolitis) occurs in as many as 5% of patients who undergo exchange transfusion. The USPSTF
concluded that evidence about the benefits and harms of screening is lacking. Thus, the USPSTF could not determine the balance of benefits and harms of
screening newborn infants to prevent chronic bilirubin encephalopathy. (2009)
9
Newborn (PKU, thyroid, hemoglobinopathies). Screening for phenylketonuria by measurement of phenylalanine level on a dried-blood spot specimen, collected by
heelstick and adsorbed onto filter paper, is recommended for all newborns before discharge from the nursery. Infants who are tested in the first 24 hours of age should
receive a repeat screening test by 2 weeks of age. Premature infants and those with illnesses optimally should be tested at or near 7 days of age, but in all cases before
newborn nursery discharge. All parents should be adequately informed regarding the indications for testing and the interpretation of PKU test results, including the
probabilities of false-positive and false-negative findings. Screening for congenital hypothyroidism with thyroid function tests performed on dried-blood spot specimens
is recommended for all newborns, optimally between days 2 and 6, but in all cases before newborn nursery discharge. Blood specimens should be collected by heelstick,
adsorbed onto filter paper, and air dried using standard technique. The choice of which thyroid function test or tests to perform is generally determined by individual
state requirements. Testing procedures and follow-up treatment for abnormal results should follow current guidelines. Care should be taken to ensure that those born at
home, ill at birth, or transferred between hospitals in the first week of life are appropriately screened before 7 days of age. Normal newborn screening results should not
preclude appropriate evaluation of infants presenting with clinical symptoms and signs suggestive of hypothyroidism. Screening newborn infants for hemoglobinopathies
with hemoglobin electrophoresis or other tests of comparable accuracy on umbilical cord or heelstick blood specimens is recommended. In geographic areas with a very
low incidence of hemoglobin disorders, selective screening of newborns may be more efficient than universal screening. (1996)
10
Testicular Cancer. The USPSTF found no new evidence that screening with clinical examination or testicular self-examination is effective in reducing mortality from
testicular cancer. Even in the absence of screening, the current treatment interventions provide very favorable health outcomes. Given the low prevalence of testicular
cancer, limited accuracy of screening tests, and no evidence for the incremental benefits of screening, the USPSTF concluded that the harms of screening exceed any
potential benefits. (2004)
11
Hearing. Children with hearing loss have increased difficulties with verbal and nonverbal communication skills, increased behavioral problems, decreased
psychosocial well-being, and lower educational attainment compared with children with normal hearing. Because half of the children with hearing loss have no
identifiable risk factors, universal screening (instead of targeted screening) has been proposed to detect children with permanent congenital hearing loss (PCHL).
There is good evidence that newborn hearing screening testing is highly accurate and leads to earlier identification and treatment of infants with hearing loss.
Good-quality evidence shows that early detection improves language outcomes. There is limited evidence about the harms of screening, with conflicting
research findings regarding anxiety associated with false-positive test results. There is limited information about the harms of treatment. Complications of
cochlear implant surgery include increased risk of meningitis; however, the overall risks of complications of screening and treatment are estimated to be small.
The USPSTF concluded that there is moderate certainty that the net benefit of screening all newborn infants for hearing loss is moderate. (2008) Routine
hearing screening of asymptomatic children beyond age 3 years is not recommended. It is recognized, however, that such testing often occurs outside the clinical
setting. When this occurs, abnormal test results should be confirmed by repeat testing at appropriate intervals, and all confirmed cases identified through screening
referred for ongoing audiologic assessment, selection of hearing aids, family counseling, psycho-educational management, and periodic medical evaluation. (1996)
12
Vision. Approximately 2% to 4% of preschool-aged children have amblyopia, an alteration in the visual neural pathway in the developing brain that can lead
to permanent vision loss in the affected eye. Amblyopia usually occurs unilaterally but can occur bilaterally. Identification of vision impairment before school
entry could help identify children who may benefit from early interventions to correct or to improve vision. The USPSTF found adequate evidence that vision
screening tools have reasonable accuracy in detecting visual impairment, including refractive errors, strabismus, and amblyopia. The USPSTF found adequate
evidence that early treatment for amblyopia, including the use of cycloplegic agents, patching, and eyeglasses, for children 3 to 5 years of age leads to improved
visual outcomes. The USPSTF found inadequate evidence that early treatment of amblyopia for children <3 years of age leads to improved visual outcomes. The
USPSTF found limited evidence regarding harms of screening, including psychosocial effects, for children ≥3 years of age. False-positive screening results may
lead to the overprescribing of corrective lenses. Adequate evidence suggests that the harms of treatment of amblyopia for children ≥3 years of age are limited to
reversible loss of visual acuity resulting from patching of the nonaffected eye. The USPSTF found inadequate evidence of the harms of screening and treatment
for children <3 years of age. The USPSTF concludes with moderate certainty that vision screening for children 3 to 5 years of age has a moderate net benefit.
The USPSTF concludes that the benefits of vision screening for children <3 years of age are uncertain and that the balance of benefits and harms cannot be
determined for this age group. (2011)
13
Speech and Language. Speech and language delay affects 5 to 8 percent of preschool children, often persists into the school years, and may be associated with
lowered school performance and psychosocial problems. The USPSTF found insufficient evidence that brief, formal screening instruments that are suitable for use in
primary care for assessing speech and language development can accurately identify children who would benefit from further evaluation and intervention. Fair evidence
suggests that interventions can improve the results of short-term assessments of speech and language skills; however, no studies have assessed long-term outcomes.
Furthermore, no studies have assessed any additional benefits that may be gained by treating children identified through brief, formal screening who would not be
identified by addressing clinical or parental concerns. No studies have addressed the potential harms of screening or interventions for speech and language delays, such as
labeling, parental anxiety, or unnecessary evaluation and intervention. Thus, the USPSTF could not determine the balance of benefits and harms of using brief, formal
screening instruments to screen for speech and language delay in the primary care setting. (2006)
14
Tuberculosis. Screening for tuberculous infection by tuberculin skin testing is recommended for all persons at increased risk of developing tuberculosis (TB).
Asymptomatic persons at increased risk include persons infected with HIV, close contacts of persons with known or suspected TB (including health care workers),
persons with medical risk factors associated with TB, immigrants from countries with high TB prevalence (e.g., most countries in Africa, Asia, and Latin America),
medically underserved low-income populations (including high-risk racial or ethnic minority populations), alcoholics, injection drug users, and residents of long-term
care facilities (e.g., correctional institutions, mental institutions, nursing homes). The frequency of tuberculin skin testing is a matter of clinical discretion. (1996)
15
Chlamydia. The USPSTF found good evidence that screening women at risk for chlamydial infection reduces the incidence of pelvic inflammatory disease and fair
evidence that community-based screening reduces prevalence of chlamydial infection. The USPSTF concludes that the benefits of screening substantially outweigh the
potential harms. The USPSTF found at least fair evidence that screening low-risk women could detect some additional cases of Chlamydia trachomatis, but concludes
that the potential benefits of screening low-risk women may be small and may not justify the possible harms. The USPSTF found at least fair evidence that screening and
treatment of women at risk for chlamydial infection improves pregnancy outcomes and concludes that the benefits of screening outweigh potential harms. The USPSTF
found fair evidence that the benefits of screening low-risk pregnant women are small and may not justify the possible harms. No direct evidence was found to determine
whether screening asymptomatic men for chlamydial infection is effective for reducing the incidence of new infections in women. The benefits and harms of screening
men cannot be determined, but the potential magnitude of benefits could be large if the effectiveness of screening men can be demonstrated. (2001)
16
Hepatitis C. The USPSTF found good evidence that screening with available tests can detect HCV infection in the general population. The prevalence of HCV
infection in the general population is low, and most who are infected do not develop cirrhosis or other major negative health outcomes. There is no evidence that
screening for HCV infection leads to improved long-term health outcomes, such as decreased cirrhosis, hepatocellular cancer, or mortality. Although there is good
evidence that anti-viral therapy improves intermediate outcomes, such as viremia, there is limited evidence that such treatment improves long-term health outcomes. The
current treatment regimen is long and costly and is associated with a high patient dropout rate due to adverse effects. Potential harms of screening include unnecessary
biopsies and labeling, although there is limited evidence to determine the magnitude of these harms. As a result, the USPSTF concluded that the potential harms of
screening for HCV infection in adults who are not at increased risk for HCV infection are likely to exceed potential benefits. The USPSTF found no evidence that
screening for HCV infection in adults at high risk leads to improved long-term health outcomes, although the yield of screening would be substantially higher in a highrisk population than in an average-risk population and there is good evidence that anti-viral therapy improves intermediate outcomes, such as viremia. There is, as yet, no
evidence that newer treatment regimens for HCV infection, such as pegylated interferon plus ribavirin, improve long-term health outcomes. There is limited evidence
from non-U.S. studies that older therapies have some long-term health benefits for patients referred for treatment, but the generalizability of these results to the U.S.
population is unknown. Of those infected with HCV, the proportion who progress to liver disease is uncertain. There is limited evidence that 10% to 20% of patients with
chronic HCV infection develop cirrhosis within 20 to 30 years after infection. There is also limited evidence that available treatments are effective in preventing cirrhosis
in patients with asymptomatic HCV infection. Potential harms of screening and treatment include labeling, adverse treatment effects, and unnecessary biopsies, although
there is limited evidence to determine the magnitude of these harms. As a result, the USPSTF could not determine the balance of benefits and harms of screening for
HCV infection in adults at increased risk for infection. (2004)
17
HIV. The USPSTF found good evidence that both standard and U.S. Food and Drug Administration (FDA)-approved rapid screening tests accurately detect HIV
infection. The USPSTF also found good evidence that appropriately timed interventions, particularly highly active antiretroviral therapy (HAART), lead to improved
health outcomes for many of those screened, including reduced risk for clinical progression and reduced mortality. Since false-positive test results are rare, harms
associated with HIV screening are minimal. Potential harms of true-positive test results include increased anxiety, labeling, and effects on close relationships. Most
adverse events associated with HAART, including metabolic disturbances associated with an increased risk for cardiovascular events, may be ameliorated by changes in
regimen or appropriate treatment. The USPSTF concluded that the benefits of screening individuals at increased risk substantially outweigh potential harms. The
USPSTF found fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV, and good
evidence that appropriately timed interventions, especially HAART, lead to improved health outcomes for some of these individuals. However, the yield of screening
persons without risk factors would be low, and potential harms associated with screening have been noted (above). The USPSTF concluded that the benefit of screening
adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation. The USPSTF found good evidence that
both standard and FDA-approved rapid screening tests accurately detect HIV infection in pregnant women and fair evidence that introduction of universal prenatal
counseling and voluntary testing increases the proportion of HIV-infected women who are diagnosed and are treated before delivery. There is good evidence that
recommended regimens of HAART are acceptable to pregnant women and lead to significantly reduced rates of mother-to-child transmission. Early detection of maternal
HIV infection also allows for discussion of elective cesarean section and avoidance of breastfeeding, both of which are associated with lower HIV transmission rates.
There is no evidence of an increase in fetal anomalies or other fetal harm associated with currently recommended antiretroviral regimens (with the exception of
efavirenz). Serious or fatal maternal events are rare using currently recommended combination therapies. The USPSTF concluded that the benefits of screening all
pregnant women substantially outweigh potential harms. (2005)
18
Syphilis. Although the USPSTF found no new direct evidence that screening for syphilis infection leads to improved health outcomes in persons at increased risk,
there is adequate evidence that screening tests can accurately detect syphilis infection and that antibiotics can cure syphilis. Screening may result in potential harms (such
as clinical evaluation of false-positive results, unnecessary anxiety to the patient, and harms of antibiotic use). The USPSTF concludes that the benefits of screening
persons at increased risk for syphilis infection substantially outweigh the potential harms. The USPSTF found observational evidence that the universal screening of
pregnant women decreases the proportion of infants with clinical manifestations of syphilis infection and those with positive serologies. The USPSTF concludes that the
benefits of screening all pregnant women for syphilis infection substantially outweigh potential harms. Given the low incidence of syphilis infection in the general
population and the consequent low yield of such screening, the USPSTF concludes that potential harms of screening (i.e., opportunity cost, false-positive tests, and
labeling) in a low-incident population outweigh the benefits. (2004)
19
Gonorrhea. Women with asymptomatic gonorrhea infection have high morbidity due to pelvic inflammatory disease, ectopic pregnancy, and chronic pelvic pain.
Pregnant women with gonorrhea infection are at risk for preterm rupture of membranes, preterm labor, and chorioamnionitis. There is fair evidence that screening tests
can accurately detect gonorrhea infection and good evience that antibiotics can cure gonorrhea infection. There is fair evidence that screening pregnant women at high
risk for gonorrhea, including women at high risk because of younger age, may prevent other complications associated with gonococcal infection during pregnancy, such
as preterm delivery and chorioamnionitis. Potential harms of screening and treatment for gonorrhea include false-positive test results, anxiety, and unnecessary antibiotic
use. There is insufficient evidence (due to a lack of studies) to quantify the magnitude of these potential harms. The USPSTF judges the magnitide of the potential harms
to be small. The USPSTF concludes that the benefits of screening women at increased risk for gonorrhea infection outweigh the potential harms. The morbidity from
undiagnosed and untreated genital gonorrhea infection is lower in men than in women. Clinical symptoms are more likely to lead to diagnosis and treatment in men; thus,
the prevalence of asymptomatic infection in men is lower. There is fair evidence that non-invasive screening tests can accurately detect gonorrhea infection and good
evidence that antibiotics cure gonorrhea infection. The USPSTF judges the magnitide of the potential harms of screening men for gonorrhea to be small. Given the low
prevalence of asymptomatic infection in men, the USPSTF could not determine the balance of benefits and harms of screening for gonorrhea infection in men at increased
risk for infection. There is a low prevalence of gonorrhea infection in the general population and consequently a low yield from screening. Thus, the USPSTF concludes
that potential harms of screening (ie, false-positive test results and labeling) in low-prevalence populations outweigh the benefits. The prevalence of gonorrhea infection
in pregnant women who are not at increased risk for infection is low. The USPSTF could not determine the balance between benefits and harms of screening for
gonorrhea in pregnant women who are not at increased risk for infection. (2005)
20
Depression. Major depressive disorder (MDD) among youth is a disabling condition that is associated with serious long-term morbidities and risk of suicide.
However, the majority of depressed youth are undiagnosed and untreated. There is adequate evidence that screening tests accurately identify MDD in
adolescents. The USPSTF found inadequate evidence that screening tests accurately identify MDD in children. Adolescents (12-18 years of age): The USPSTF
found adequate evidence that treatment in adolescents with selective serotonin reuptake inhibitors (SSRIs), psychotherapy, and combined therapy (SSRIs and
psychotherapy) results in decreases in MDD symptoms. Children (7-11 years of age): The USPSTF found inadequate evidence to support the benefits of
treatment in children. SSRIs (fluoxetine) reduce MDD symptoms in children; however, there are limited data on the benefits of psychotherapy and the benefits of
psychotherapy plus SSRIs in children. For adolescents (12-18 years of age) there is convincing evidence that there are harms of SSRIs (risk of suicidality [i.e.,
suicide ideation, preparatory acts, or suicide attempts]) in adolescents. Limited evidence exists regarding the harms of combining SSRIs and psychotherapy.
However, there is inadequate evidence about the harms of screening and psychotherapy in adolescents, which are probably small. For children (7-11 years of
age) SSRIs (fluoxetine) demonstrated harms in children (risk of suicidality); however, there is limited evidence on the harms of psychotherapy and on the harms
of combining psychotherapy and SSRIs (fluoxetine) in children. There is also limited evidence about the harms of screening children. The USPSTF judged that
the overall evidence is inadequate regarding the harms of screening and treatment in children. The USPSTF concluded that: In adolescents (12-18 years of age),
there is moderate certainty that the net benefit of psychotherapy is moderate. In children (7-11 years of age), the evidence is lacking, and the balance of benefits
and harms of psychotherapy cannot be determined. (2009)
21
Suicide. The USPSTF found no evidence that screening for suicide risk reduces suicide attempts or mortality. There is limited evidence on the accuracy of screening
tools to identify suicide risk in the primary care setting, including tools to identify those at high risk. The USPSTF found insufficient evidence that treatment of those at
high risk reduces suicide attempts or mortality. The USPSTF found no studies that directly address the harms of screening and treatment for suicide risk. As a result, the
USPSTF could not determine the balance of benefits and harms of screening for suicide risk in the primary care setting. (2004)
22
Flouride Supplement. The USPSTF found fair evidence that, in preschool children with low fluoride exposure, prescription of oral fluoride supplements by primary
care clinicians leads to reduced dental caries. The USPSTF concluded that the benefits of caries prevention using oral fluoride supplementation outweigh the potential
harms of dental fluorosis, which in the United States are primarily observed as a mild cosmetic discoloration of the teeth. (2004)
23
Iron Supplement. Iron deficiency anemia is associated with psychomotor and cognitive abnormalities in children. Iron deficiency anemia in pregnancy has been
associated with increased risk for low birth weight, preterm delivery, and perinatal mortality. Recent studies suggest that maternal iron deficiency anemia may be
associated with postpartum depression and poor performance on mental and psychomotor tests in offspring. The prevalence of iron-deficiency anemia has remained stable
over the last decade in the general U.S. population and continues to be greatest among minority and poor children. A validated risk assessment tool to guide primary care
physicians in identifying individuals who would benefit from iron supplementation has not been developed. The USPSTF found fair evidence that iron supplementation
(e.g., iron-fortified formula or iron supplements) may improve neurodevelopmental outcomes in children at increased risk for iron deficiency anemia. The USPSTF found
poor evidence (poor quality and conflicting studies) that iron–fortified formula or supplementation improves neurodevelopmental outcomes in children aged 6 to 12
months if they are not at increased risk for iron deficiency anemia. The USPSTF found poor evidence (poor quality studies) that iron supplementation may improve health
outcomes in non-anemic pregnant women. The USPSTF found fair evidence that oral iron supplementation increases the risk for unintentional overdose and
gastrointestinal symptoms. Given appropriate protection against overdose, these harms are small. There is poor evidence (poor quality studies) that iron supplementation
for non-anemic pregnant women results an increased risk for harms. The USPSTF concludes that the moderate benefits of iron supplementation in asymptomatic children
aged 6 to 12 months who are at increased risk for iron deficiency anemia outweigh the potential harms. The USPSTF was unable to determine the balance between the
benefits and harms of iron supplementation in children aged 6 to 12 months who are at average risk for iron deficiency anemia, and of iron supplementation in nonanemic pregnant women. (2006)
24
Folic Acid. Folic acid supplementation at a dose of 4 mg/day beginning 1-3 months prior to conception and continuing through the first trimester is recommended for
women planning pregnancy who have previously had a pregnancy affected by a neural tube defect, to reduce the risk of recurrence. It is also recommended that all
women planning pregnancy take a daily multivitamin or multivitamin-multimineral supplement containing folic acid at a dose of 0.4-0.8 mg, beginning at least 1 month
prior to conception and continuing through the first trimester, to reduce the risk of neural tube defects. Taking a daily multivitamin containing 0.4 mg of folic acid is also
recommended for all women capable of becoming pregnant, to reduce the risk of neural tube defects in unplanned pregnancies ("B" recommendation). Women taking
drugs that interfere with folate metabolism (e.g., methotrexate, pyrimethamine, trimethoprim, phenytoin), women at increased risk of vitamin B12 deficiency (e.g., vegans
or persons with AIDS), and those with epilepsy whose seizures are controlled by anticonvulsant therapy, should consult with their clinician regarding potential risks and
benefits prior to considering folic acid supplementation. There is currently insufficient evidence to recommend for or against counseling women planning or capable of
pregnancy to increase their dietary folate consumption to 0.4 mg/day as an alternative to taking multivitamins with folic acid. Offering counseling to increase dietary
folate intake to women who do not wish to take folic acid supplements may be recommended on other grounds, including low risk, low cost, and likely benefit. (1996)
25
Opthalmia Neonatorum. The USPSTF recommends that all newborns receive prophylaxis; however, some newborns are at increased risk for gonococcal
ophthalmia neonatorum. Newborns at increased risk include those with a maternal history of sexually transmitted infections, substance abuse, or no prenatal care.
There is convincing evidence that blindness due to gonococcal ophthalmia neonatorum has become rare in the United States since the implementation of
universal prophylaxis of newborns. There is convincing evidence that universal prophylaxis of newborns is not associated with serious harms. The USPSTF
concluded that there is high certainty that the net benefit is substantial for topical ocular prophylaxis for all newborns for the prevention of gonococcal
ophthalmia neonatorum. (2011)
26
Tobacco Use. The USPSTF found adequate evidence that behavioral counseling interventions, such as face-to-face or phone interaction with a health care
provider, print materials, and computer applications, can reduce the risk of smoking initiation in school-aged children and adolescents. The USPSTF found no
evidence on the harms of behavioral interventions to prevent tobacco use; however, the magnitude of these potential harms is probably small to none. The
USPSTF concludes with moderate certainty that primary care–relevant behavioral interventions to prevent tobacco use in school-aged children and adolescents
have a moderate net benefit. Evidence on the effectiveness of cessation interventions delivered in primary care settings to school-aged children and adolescents
who have experimented with smoking or are regular smokers is limited. The USPSTF examined the evidence on behavioral interventions to promote smoking
cessation in children and adolescents who were classified as smokers. Few studies targeted regular, established smokers or stratified findings by length or amount
of smoking (such as experimenters vs. established smokers). Although evidence on the effectiveness of primary care–relevant interventions in reducing smoking
in children and adolescents is limited, some evidence from other literature shows that school- and community-based behavioral counseling programs can promote
smoking cessation in adolescent smokers. No medications are currently approved by the U.S. Food and Drug Administration for tobacco cessation in children
and adolescents. Two studies that evaluated behavioral interventions plus medication (sustained-release bupropion alone or combined with nicotine replacement
therapy) showed no statistically significant benefit from the medication . Evidence on complementary and alternative medicine, such as acupuncture, for
smoking cessation in children and adolescents is not available, and such interventions have demonstrated no long-term benefits in adults. (2013)
27
Alcohol Misuse. The evidence on screening for alcohol misuse and brief behavioral counseling interventions in the primary care setting for adolescents is
insufficient, and the balance of benefits and harms cannot be determined. (2013)
28
Healthy Diet. The USPSTF found fair evidence that brief, low- to medium-intensity behavioral dietary counseling in the primary care setting can produce small-tomedium changes in average daily intake of core components of an overall healthy diet (especially saturated fat and fruit and vegetables) in unselected patients. The
strength of this evidence, however, is limited by reliance on self-reported diet outcomes, limited use of measures corroborating reported changes in diet, limited followup
data beyond 6 to 12 months, and enrollment of study participants who may not be fully representative of primary care patients. In addition, there is limited evidence to
assess possible harms. though community-based studies have evaluated measures to reduce dietary fat intake in children, no controlled trials of routine behavioral dietary
counseling for children or adolescents in the primary care setting were identified. (2003)
29
Physical Activity. The USPSTF found insufficient evidence to determine whether counseling patients in primary care settings to promote physical activity leads to
sustained increases in physical activity among adult patients. Controlled trials of physical activity counseling in adult primary care patients were of variable quality and
had mixed results. There were no completed trials with children or adolescents that compared counseling with usual care practices. Data on the feasibility and potential
harms of routine physical activity counseling in primary care settings are limited. As a result, the USPSTF could not determine the balance of potential benefits and harms
of routine counseling to promote physical activity in adults. The USPSTF reviewed only the literature on the effectiveness of primary care counseling to promote physical
activity. It did not review the evidence for the effectiveness of physical activity to reduce chronic disease morbidity and mortality, which has been well documented in
other recent reviews, or review evidence of counseling in other settings. (2002)
30
Obesity. Approximately 15 percent of children and adolescents aged 6 to 19 years are overweight and are at risk for diabetes, elevated blood lipids, increased blood
pressure and their sequelae, as well as slipped capital femoral epiphysis, steatohepatitis, sleep apnea, and psychosocial problems. The USPSTF found fair evidence that
body mass index (BMI) is a reasonable measure for identifying children and adolescents who are overweight or are at risk for becoming overweight. There is fair
evidence that overweight adolescents and children aged 8 years and older are at increased risk for becoming obese adults. The USPSTF found insufficient evidence for
the effectiveness of behavioral counseling or other preventive interventions with overweight children and adolescents that can be conducted in primary care settings or to
which primary care clinicians can make referrals. There is insufficient evidence to ascertain the magnitude of the potential harms of screening or prevention and treatment
interventions. The USPSTF was, therefore, unable to determine the balance between potential benefits and harms for the routine screening of children and adolescents for
overweight. (2005)
31
Skin Cancer Prevention. Behavior change interventions are aimed at techniques shown to be effective in reducing ultraviolet (UV) radiation exposure.
Ultraviolet radiation comes from exposure to the sun during midday hours and from artificial sources of UV light (such as indoor tanning). Sun-protective
behaviors include the use of broad-spectrum sunscreen with a sun-protection factor of 15 or greater, wearing hats or other shade-protective clothing, avoiding the
outdoors during midday hours (10 a.m. to 3 p.m.), and avoiding indoor tanning. Utilizing all behaviors is important to minimizing risk. Epidemiologic evidence
(3) links ultraviolet radiation exposure with incidence of all three types of skin cancer. The USPSTF found convincing evidence linking UV radiation exposure
during childhood and youth to a moderately increased risk for skin cancer later in life; for adults, adequate evidence links UV radiation exposure to a small
increase in risk for skin cancer. Persons with fair skin, light hair and eye color, or freckles or who sunburn easily are at increased risk for skin cancer (1). Most
studies of interventions to increase sun-protective behaviors have been limited to populations with a fair skin type. For children, adolescents, and young adults
(persons aged 10 to 24 years), the USPSTF found adequate evidence that counseling interventions that are available in a primary care setting or are referable
from primary care can moderately increase the use of sun-protective behaviors. For adults older than 24 years, the USPSTF found inadequate evidence to
determine the effect of counseling on the use of sun-protective behaviors. The USPSTF found adequate evidence that no appreciable harms are related to
counseling or sun-protective behaviors in young persons or adults. Theoretical concerns about sun-protective behaviors include the risk for vitamin D deficiency
in adults living in northern latitudes, but little evidence supports this hypothesis. The USPSTF concluded that for children, adolescents, and young adults aged
10 to 24 years with fair skin, there is moderate certainty that counseling has a moderate net benefit. The USPSTF concluded that for adults older than 24 years,
evidence of the benefits of counseling is sparse and of unknown clinical significance; therefore, the balance of benefits and harms cannot be determined. (2012)
32
Dental Caries. The USPSTF found no validated risk-assessment tools or algorithms for assessing dental disease risk by primary care clinicians and little evidence that
primary care clinicians are able to systematically assess risk for dental disease among preschool-aged children. The USPSTF further found little evidence that either
counseling of parents or referring high-risk children to dental care providers results in fewer caries or reduced dental disease. Thus, the USPSTF concluded there is
insufficient evidence to determine the balance between the benefits and harms of routine risk assessment to prevent dental disease among preschool children. (2004)
33
Sexually Transmitted Infections. Primary care clinicians and teams can identify adolescents and adults who are at increased risk. There is convincing evidence
that high-intensity behavioral counseling interventions targeted to sexually active adolescents and adults at increased risk for STIs reduce the incidence of STIs.
These results were found 6 and 12 months after counseling took place. The USPSTF has identified the absence of studies and evidence on behavioral counseling
interventions directed towards adults not at increased risk for STIs and non-sexually-active adolescents as a critical gap in the literature. No evidence of
significant behavioral or biological harms resulting from behavioral counseling about risk reduction has been found. The USPSTF concluded that the potential
harms of counseling are no greater than small. The USPSTF concluded that there is moderate certainty that high-intensity behavioral counseling has a moderate
net benefit for sexually active adolescents and for adults who are at increased risk for STIs. The USPSTF concluded that the evidence is currently insufficient to
assess the balance of benefits and harms of behavioral counseling for non-sexually active adolescents and for adults who are not at increased risk for STIs. (2008)
34
Child Maltreatment. There is inadequate evidence that primary care interventions can prevent maltreatment among children who do not already have signs or
symptoms of such treatment. Reasons for this conclusion include significant heterogeneity in study methods and interventions. There is also inconsistent and
limited evidence on outcomes or how they were measured. Although there are numerous concerns about the possible harms of interventions for child
maltreatment, evidence of these harms is limited. The USPSTF concluded that the evidence is limited and inconsistent, and is therefore insufficient to determine
the balance of benefits and harms of interventions in primary care to prevent child maltreatment among children without signs or symptoms of maltreatment.
(2013)