* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PV Diagrams
Equation of state wikipedia , lookup
Heat transfer physics wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
History of thermodynamics wikipedia , lookup
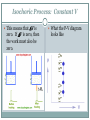
PV Diagrams THERMODYNAMICS A Generic Thermodynamic Process Start with some internal energy due to a starting T, P, and V Internal Energy T1 U1 P 1 V1 Q Heat is added or taken away, work is done on or by the gas Thermodynamic Process W Internal Energy T2 U2 P 2 V2 You end up with an new amount of internal energy, with new T, P, and V Many engines that do work involve pistons and cylinders. A cylinder is tube and a piston is a solid cylindrical device that exactly fits into the cylinder so that there is very little gap between the piston and the side of the cylinder. piston cylinder Pistons and Cylinders The piston can move up and down in the cylinder. Inside the cylinder, under the piston, is a gas. The gas cannot escape around the piston. Push down on the piston and the gas is compressed into a smaller volume. Pull up on the piston and the gas expands into a larger volume. The motion of the piston involves work. If you do work on the piston, you make it move. If the gas expands or is compressed causing the piston to move, then work is done on the piston (the gas is doing the work). isobaric process: constant P If you add heat to the cylinder, the gas expands and pushes the piston upwards, doing work on it. We make a major assumption – that the pressure stays constant. This is actually reasonable. The area on the top of the piston doesn’t change, so the force exerted on it by the atmosphere is constant. When the piston rises to some position, the force pushing down is still the same, so the force inside pushing up must be the same as well. Since this force comes from the pressure, the pressure must still be the same. y Gas expands at constant pressure moving piston a distance of y isobaric process: constant P What a P-V Diagram would look like How to Find the Amount of Work Done The piston has moved a distance of y. A force pushed the piston upward. Work happens when a force causes an object to move. So can we figure out how much work was done on the piston? Well, we know that pressure is the force divided by the area it acts on: P=F/a The force exerted by the pressure is equal to: F = PA The definition of work is: W=Fd So, plugging in the value of the force, we get: W Fd PA d PAd We know that the area of this cylinder multiplied by its height is its change in volume: Therefore, plugging this in for Ad we get: So W PV W PAd P V PV V Ad Sign of work How to Find the Amount of Work Done The area under the curve is equal to the work that is done. This is also true for any graph of pressure vs volume. The area under the curve is always equal to the work. W PV Work Done Using a P-V Diagram note that in a cyclic process the gas is taken through a cycle of operations and brought back to the original state. If the cycle is clockwise as in the present case, work is done by the gas. So the work is negative. Further, the area enclosed by the closed curve gives the work done during one cycle. Isochoric Process: Constant V This means that V is zero. If V is zero, then the work must also be zero. What the P-V diagram looks like Isothermal Process: Constant T During this process heat can enter and leave the gas but the total energy in the gas will not change. ∆U=ZERO U Q W 0 Q W Q W What the P-V diagram looks like Adiabatic Processes: Q 0 In an adiabatic process, heat is neither added or taken away from the system. This means that the first law can be simplified: U Q W U W What this means is that work is done by the system at the expense of internal energy. Adiabatic Processes Adiabatic processes are very common. There are two simple ways to have one take place: 1. The system can be insulated so that heat can neither enter nor leave. Joule’s “heat equivalent” experiment was adiabatic. The tub of water was insulated so the water would not absorb heat from its surroundings. Instead the work done on the water by the paddles increased the internal energy of the system - the temperature went up. 2. The other way to have an adiabatic process (this is actually called a “near adiabatic process”) is to have it happen very quickly. The process happens so fast that there is no time for heat to be transferred. The combustion of gasoline in an engine is considered to be adiabatic because each combustion step happens in a very short time – a few hundredths (or less) of a second. Adiabatic Processes In an adiabatic process the following can happen: 1. a gas that is adiabatically expanded will lose internal energy (U ) and become cooler. 2. a gas that is adiabatically compressed will gain U and become warmer. Compress air from your lungs by puckering your lips. When it leaves your mouth, it expands adiabatically, thus it cools Sample Problems One mole of monatomic ideal gas is enclosed under a frictionless piston. A series of processes occur, and eventually the state of the gas returns to its initial state with a P-V diagram as shown below. Answer the following in terms of P0, V0, and R. 1. Find the temperature at each vertex. 2. Find the change in internal energy for each process. 3. Find the work by the gas done for each process. 1. Find the temperature at each vertex. Use the gas law to find the temperatures at A, B, C 2. Find the change in internal energy for each process. Since the internal energy depends only on temperature, the change in internal energy for each process depends only on the temperature difference that occurs during the process: 3. Find the work by the gas done for each process. To find the work done by the gas, find the area under each segment, remembering the sign convention.