* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Building Blocks
Survey
Document related concepts
Bohr–Einstein debates wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
Elementary particle wikipedia , lookup
Matter wave wikipedia , lookup
Molecular orbital wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Wave–particle duality wikipedia , lookup
Chemical bond wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Atomic orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron configuration wikipedia , lookup
Transcript
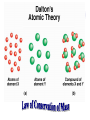
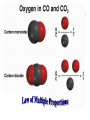
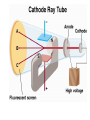
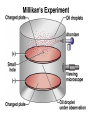
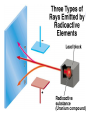
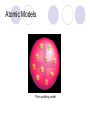
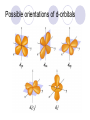
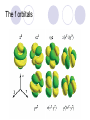
Chemical Building Blocks Atomic theories, models and electronic structure QUIZ: ½ sheet of paper An analyst measured a 3.3 x 10-2 L sample and found it weighed 385 mg. Report its density in g/mL. (Proper SF) Give an example of the ff. A colloid A pure substance An intensive property An extensive property A chemical property Parts of the atom (3pts., bonus of 1 pt. each for other subatomic particles) History of the Atomic Theory Dalton’s Atomic Theory 1. Matter is composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. 3. Compounds are composed of atoms of different elements combined in fixed proportions. 4. Chemical reactions only involve the rearrangement of atoms. Atoms are not created or destroyed in chemical reactions. Cathode Ray Tube Cathode ray tube television Radioactivity (Uranium compound) Atomic Models Plum pudding model Rutherford’s gold foil experiment Rutherford’s atomic model Discovery of the Neutron Chadwick’s equation: Parts of the atom Protons Electrons Neutrons Atomic number and mass number isotopes Test Yourself! How many neutrons are there in the 90Sr nucleus? A rubidium isotope has 50 neutrons. What is its mass no.? How many neutrons does 90Mo have? How many neutrons are in bromine-81? Which of the following isotopes are of the same element? Name the isotopes. 90 37 X 90 35 X 88 37 X 88 38 X 93 38 X Electromagnetic spectrum Photoelectric effect Einstein Continuous vs. Line Spectra Bohr’s Model Allowed energy states Energy is quantized (flame test) Heisenberg’s uncertainty principle An observer always has an effect on the observed Location and momentum of electron Schrodinger’s wave equations: quantum numbers (n, l, m, ms) Orbitals Possible orientations of d-orbitals The f orbitals Orbital energy levels in hrdrogen atom Energy only depends on principal quantum number n n=3 n=2 n=1 Orbital energy levels in multi-electron atom Energy depends on n and l n=3 l = 2 n=3 l = 0 n=2 l = 0 n=1 l = 0 n=3 l = 1 n=2 l = 1 Outermost subshell containing electrons Additional exercises For n=4, what are the possible values of l and ml? Which of the following are permissible sets of quantum numbers for an electron in a hydrogen atom? (n,l,ml) (2,2,-2) (4,3,-1) (1,0,0) (6,3,-4)