* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Update for Nurse Anesthetists - American Association of Nurse
Neuropsychopharmacology wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Discovery and development of dipeptidyl peptidase-4 inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Methylene blue wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
History of general anesthesia wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
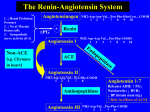
AANA Journal Course 1 Update for Nurse Anesthetists Pathophysiology and Management of AngiotensinConverting Enzyme Inhibitor–Associated Refractory Hypotension During the Perioperative Period Andrea Thoma, CRNA, MS Hypertension is a common chronic condition in many patients requiring anesthesia. Pharmacologic therapy is a mainstay of treatment for hypertension, with angiotensin-converting enzyme (ACE) inhibitors being a frequently prescribed class of drugs. The American College of Cardiology and American Heart Association 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery provide information on many drug classes used in the treatment of hypertension; noticeably absent is a guideline for ACE inhibitors. Literature demonstrates that practice standards vary on whether ACE inhibitor regimens are continued or withheld during the preoperative period. When ACE inhibitor therapy is continued in patients undergoing general anesthesia, varying degrees of hypotension can be seen depending on confounding Objectives At the completion of this course, the reader should be able to: 1. Describe the factors that control short-term and long-term blood pressure regulation. 2.Identify the therapeutic effects and side effects of ACE inhibitors. 3.Understand the two types of hypotension associated with the preoperative use of ACE inhibitors. 4.Explain the pathophysiology of vasoplegic syndrome. 5.Discuss available treatment options for vasoplegic syndrome. Introduction As many as 60 million Americans have been diagnosed with hypertension (blood pressure ≥ 140/90 mm Hg).1 The prevalence of hypertension increases with advanced patient variables and the type of surgical procedure. In some instances, this hypotension can be refractory to traditional interventions such as administration of a fluid bolus, ephedrine, or phenylephrine. Vasopressin and methylene blue have been found to be effective treatments for ACE inhibitor–associated refractory hypotension. With the prevalence of hypertension and use of ACE inhibitors, anesthesia providers are likely to encounter refractory hypotension of this nature. The absence of guidelines regarding ACE inhibitors in the perioperative period contributes to a lack of consistency in practice. Keywords: Angiotensin-converting enzyme (ACE) inhibitor, methylene blue, refractory hypotension, vasoplegic syndrome, vasopressin. age. There is often a delay in diagnosis and treatment because of a lack of overt signs and symptoms associated with hypertension. The development of ischemic heart disease, congestive heart failure, and atherosclerosis leading to stroke and renal failure can occur with long-standing hypertension. Pharmacologic therapy with various classes of antihypertensive drugs is a mainstay of treatment when diet and exercise do not lower blood pressure to acceptable values. Angiotensin-converting enzyme (ACE) inhibitors are commonly prescribed antihypertensives. This class of drugs is advantageous for control of blood pressure because of its many other therapeutic effects often of value to patients with hypertension.2 When ACE inhibitors are taken within 8 to 24 hours of general anesthesia, intraoperative hypotension is more likely.3 This hypotension can occur following the induction of anesthesia and AANA Journal Course No. 33 (Part 1): AANA Journal course will consist of 6 successive articles, each with objectives for the reader and sources for additional reading. At the conclusion of the 6-part series, a final examination will be published on the AANA website and in the AANA Journal. This educational activity is being presented with the understanding that any conflict of interest on behalf of the planners and presenters has been reported by the author(s). Also, there is no mention of off-label use for drugs or products. www.aana.com/aanajournalonline AANA Journal April 2013 Vol. 81, No. 2 133 can occasionally be refractory to standard treatments such as administration of a fluid bolus, ephedrine, or phenylephrine.3-7 In addition, continued preoperative use of ACE inhibitors can be associated with a more profound and persistent hypotension in surgical patients undergoing cardiopulmonary bypass (CPB). This form of ACE inhibitor–associated refractory hypotension is a type of a vasodilatory shock known as vasoplegic syndrome (VS).8-10 Vasopressin and methylene blue have emerged as treatment options that are effective in the treatment of VS.9,11-13 Bolus doses of vasopressin agonists have been used successfully for the treatment of ACE inhibitor–associated hypotension following induction of anesthesia.4-7 Anesthesia providers are certain to encounter hypertensive patients being treated with ACE inhibitors. Unfortunately, unequivocal guidelines regarding the preoperative administration of ACE inhibitors and perioperative management of ACE inhibitor–associated refractory hypotension do not exist, most likely because of a paucity of research and the variability with which ACE inhibitor– associated refractory hypotension manifests, especially as it relates to its incidence and severity. Owing to the lack of definitive guidelines, anesthesia providers must understand the pathophysiology of hypertension and the implications related to ACE inhibitors and anesthesia. Physiology of Short-term Blood Pressure Regulation Knowledge of mechanisms that contribute to hemodynamic stability is necessary to understand the dysfunction that occurs when hypertension exists. Regulation of blood pressure is required to maintain homeostasis and involves short- and long-term regulatory mechanisms. An area of the brain termed the vasomotor center governs primary control of the cardiovascular system. The vasomotor center regulates the autonomic nervous system, thus influencing vasoconstriction and vasodilation.1 Short-term regulation involves neural reflexes and hormones. These adaptations respond to acute decreases in mean arterial pressure (MAP) and restore MAP to 50 to 120 mm Hg within 30 minutes.1 There are 6 types of reflexes that influence short-term regulation of blood pressure: (1) atrial stretch reflex, (2) baroreceptor reflex, (3) chemoreceptor reflexes, (4) Cushing reflex, (5) celiac reflex, and (6) oculocardiac reflex. The atrial stretch reflex, also known as the Bainbridge reflex, occurs when the atria are stretched in response to hypervolemia.14 Reflex stimulation causes vascular smooth muscle vasodilation, increased heart rate, decreased systemic vascular resistance (SVR), and decreased MAP.14 Associated with the atrial stretch reflex is the release of atrial natriuretic factor, which causes dilation of renal vasculature leading to increased glomerular filtration and decreased secretion of antidiuretic 134 AANA Journal April 2013 Vol. 81, No. 2 hormone (ADH), resulting in diuresis and a further decrease in MAP.1 Baroreceptors in the aortic arch and carotid sinus also contribute to short-term regulation through activation or inhibition of neural impulses to the vasomotor center in the presence of hypotension or hypertension respectively.14 Over time, baroreceptors adapt to higher blood pressures, making this reflex less effective in longterm regulation.1 In addition, the baroreceptor reflex is blunted by the use of volatile anesthetics.1 Central chemoreceptors in the medulla and peripheral chemoreceptors in the carotid and aortic bodies contribute mostly to respiratory system regulation, but also have a lesser role in the short-term regulation of MAP.15 Stimulation of central chemoreceptors is associated with increased levels of hydrogen ions in the blood, and stimulation for peripheral chemoreceptors is primarily due to hypoxia.15 The circulatory response seen with stimulation of chemoreceptors is an increase in sympathetic nervous system activity accounting for the increase in MAP.15 The Cushing reflex is also a short-term regulatory mechanism that occurs with increases in intracranial pressure.1 With increased intracranial pressure, the sympathetic nervous system is stimulated, thereby increasing MAP in an effort to maintain cerebral perfusion pressure. Finally, the celiac and oculocardiac reflexes cause hypotension and reflex bradycardia when traction is applied to the thoracic or abdominal mesentery and extraocular muscles, respectively.1 Also contributing to short-term regulation are hormones, notably norepinephrine, epinephrine, ADH, and angiotensin II. Although hormones have a slower onset compared with the neural reflexes, they are still capable of restoring MAP to normal limits within 30 minutes.1 The central nervous system and adrenal medulla are responsible for releasing norepinephrine and epinephrine in response to sympathetic stimulation, resulting in vasoconstriction and increased MAP.1 Two additional hormones, ADH and angiotensin II, further contribute to short- and long-term regulation. As mentioned for its role in the atrial stretch reflex, ADH is a potent hormone released from the posterior pituitary gland that increases MAP through vasoconstriction.1 The role of ADH in long-term regulation is through water reabsorption in the collecting ducts of the kidney, thus promoting fluid retention.16 Angiotensin II, a powerful vasoconstrictor, is part of the renin-angiotensin-aldosterone system (RAAS), which contributes to regulation of MAP and blood volume.16 Physiology of Long-term Blood Pressure Regulation The goal of long-term blood pressure regulation is euvolemia.1 The kidneys are the predominant organ www.aana.com/aanajournalonline Figure 1. Renin-Angiotensin-Aldosterone System Abbreviations: MAP, mean arterial pressure; Na+, sodium. (Adapted from Cheung.17) through which euvolemia is achieved, with the RAAS being of utmost importance. When hypotension, sodium depletion, or sympathetic nervous stimulation occurs, the juxtaglomerular cells of the kidney secrete the enzyme renin.16 Renin interacts with circulating angiotensinogen, a serum glycoprotein produced by the liver, to form the hormone angiotensin I.16 Angiotensin I is biologically inactive, with the majority of angiotensin I converted to angiotensin II in the lungs by ACE.16 Angiotensin II is not only a potent vasoconstrictor, but also a stimulus for the production and release of aldosterone from the adrenal cortex.16 Aldosterone, the primary mineralocorticoid of the body, promotes sodium reabsorption, water retention, and potassium secretion in the distal tubules and collecting ducts of the kidney, thus increasing MAP16 (Figure 1). The complex interactions of blood pressure regulation make identifying a specific dysfunction as the primary cause of hypertension difficult. An immense amount of intertwined physiology exists between and within short- and long-term regulatory mechanisms. Owing to this complexity, a variety of pharmacologic agents with differing mechanisms of action can be used to treat hypertension. www.aana.com/aanajournalonline ACE Inhibitor Pharmacology Multiple classes of drugs exert their effects by inhibiting a specific phase of the RAAS; ACE inhibitors are one of these classes. The primary mechanism of action of ACE inhibitors is competitive antagonism of the conversion of angiotensin I to angiotensin II.17 Another proposed contributing mechanism of action is the inhibition of bradykinin catabolism.17 Bradykinin is a peptide that promotes the release of nitric oxide (NO) from endothelial cells, resulting in vasodilation of vascular smooth muscle and thereby contributing to a decrease in MAP.17 The ending “-pril” in the generic form makes drugs of this class easily recognizable. With the exception of fosinopril, which is metabolized by the liver, all other ACE inhibitors undergo primary renal excretion.17 The average half-life of commonly prescribed ACE inhibitors is approximately 10 hours, which explains why withholding ACE inhibitors for 8 to 24 hours preoperatively tends to decrease the incidence of hypotension.3 The ACE inhibitors are commonly prescribed for their many additional therapeutic effects, including stabilization of atherosclerotic plaques, inhibition of left ventricular hypertrophy, prevention of myocardial AANA Journal April 2013 Vol. 81, No. 2 135 remodeling, decreased SVR, decreased platelet aggregation, decreased intraglomerular pressure, decreased fluid retention, increased insulin sensitivity, and antiinflammatory properties.2 Another advantage of ACE inhibitors is the minimal development of tolerance to their antihypertensive effect.18 Unlike other classes of antihypertensive drugs, ACE inhibitors do not cause orthostatic hypotension, rebound hypertension with missed doses, or insomnia.18 The ACE inhibitors are not without side effects, with a persistent dry cough a common occurrence. The cause of cough is proposed to be the result of increased levels of bradykinin.19 Other side effects include hypotension, especially with the first dose; angioedema; rash; leukopenia; renal impairment; and an altered sense of taste.17 It should be noted that upper airway angioedema can be life threatening. Recognized drug interactions with ACE inhibitors include hyperkalemia when combined with a potassium-sparing diuretic, increased risk of renal complications when combined with nonsteroidal antiinflammatory drugs, and decreased antihypertensive effect when oral contraceptives are also prescribed.17 If a woman taking an ACE inhibitor becomes pregnant, an alternative class of antihypertensives that is not teratogenic should be prescribed.17 ACE inhibitors should be avoided in volume-depleted patients and in the presence of decreased renal perfusion because of the potential for renal complications.20 Anesthesia providers should recognize ACE inhibitors when they are part of a patient’s medication regimen and inquire about the timing of the last dose. Postinduction ACE Inhibitor–Associated Hypotension Patients with hypertension have been observed to have an increased incidence of hypotension and labile blood pressure during general anesthesia. It remains controversial whether intraoperative hypotension is more frequently observed in hypertensive patients treated with ACE inhibitors compared with other antihypertensive drugs.3,21 Two types of hypotension associated with the preoperative use of ACE inhibitors within 8 to 24 hours of general anesthesia have been documented. They include intraoperative hypotension following induction of anesthesia or a more profound and persistent hypotension in patients undergoing surgery that requires CPB. Some literature supports that hypotension is more likely to occur after induction among patients who continue their ACE inhibitor preoperatively and even more likely when antihypertensives from multiple drug classes or diuretics are taken.3,22 In some studies, ACE inhibitor–associated hypotension following induction was refractory to standard treatments.3-7 After the 30-minute postinduction phase, the incidence of intraoperative hypotension was similar whether an ACE inhibitor was withheld or taken 136 AANA Journal April 2013 Vol. 81, No. 2 the day of surgery.3 The sympathetic nervous system, the RAAS, and the vasopressin system are 3 endogenous vasopressor systems in the body.4 When one of these systems is inhibited, the body is able to compensate and effectively prevent hypotension. The cause of ACE inhibitor–associated refractory hypotension is the inhibition of the sympathetic nervous system by anesthetic agents and also the blockade of the RAAS by ACE inhibitors.4 With 2 of the endogenous vasopressor systems inhibited, the likelihood of hypotension increases and treatment of hypotension can be more difficult. Since the vasopressin system is the only functioning endogenous vasopressor system, this explains why vasopressin and vasopressin agonists are effective in treating ACE inhibitor–associated refractory hypotension. The American College of Cardiology and American Heart Association 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery detail specific guidelines for continuing or withholding several classes of antihypertensives in the perioperative period.23 Noticeably lacking is an unequivocal statement for the perioperative use of ACE inhibitors. Some recommendations suggest withholding ACE inhibitors the day of surgery and resuming therapy once normal intravascular volume has been restored to prevent renal dysfunction.23 Withholding of ACE inhibitors preoperatively remains controversial because not all patients develop ACE inhibitor–associated hypotension during anesthesia.21,24,25 Pathophysiology of Vasoplegic Syndrome Refractory hypotension associated with ACE inhibitors can be seen in patients undergoing surgery that requires CPB, although this topic remains controversial in the literature as well.25 Vasoplegic syndrome is a form of vasodilatory shock characterized by hypotension and decreased central venous pressure, decreased SVR, and normal cardiac output after the discontinuation of CPB.13 A distinguishing characteristic of VS is the inability to correct hypotension after adequate fluid resuscitation and administration of vasopressors.10 The incidence of VS following cardiac surgery is approximately 9% to 10%.13 Prolonged CPB times, inadequate left ventricular function, and preoperative administration of ACE inhibitors, intravenous heparin, and calcium channel blockers contribute to the development of VS.9,13 Specifically, ACE inhibitors have been reported to increase the incidence of VS by up to 44%13 Three possible mechanisms have been theorized to explain the pathophysiology of VS: (1) activation of adenosine triphosphate–sensitive potassium channels, (2) activation of the inducible form of NO synthase, and (3) ADH deficiency.10 Adenosine triphosphate–sensitive potassium channels in vascular smooth muscle are activated, making the cells more negative (hyperpolarized), www.aana.com/aanajournalonline thus preventing the entry of calcium into the cells.10 Without an influx of calcium, vascular smooth muscle contraction cannot occur. A second contributing factor to VS is activation of the inducible form of NO synthase, the enzyme responsible for catalyzing the reaction to form NO.10 When NO is synthesized and released, the enzyme guanylate cyclase is activated, thereby causing an increase in the second messenger cyclic guanosine monophosphate.10 In turn, cyclic guanosine monophosphate results in vasodilation of vascular smooth muscle. The third proposed mechanism for the hypotension seen with VS is a deficiency of ADH.10 In the early stages of VS, circulating levels of ADH are elevated.10 As the condition progresses, ADH levels decrease, possibly due to depleted hormone stores in the posterior pituitary gland or as a result of prolonged baroreceptor reflex stimulation.10 In addition, bradykinin contributes to ACE inhibitor– associated hypotension when CPB is used. As previously discussed, bradykinin results in vasodilation of vascular smooth muscle. Circulating levels of bradykinin are elevated with the use of ACE inhibitors and are further increased during CPB because the lungs are not functional and therefore are unable to break down the peptide.13 Treatment of ACE Inhibitor–Associated Refractory Hypotension Vasopressin and methylene blue have been documented as treatment options for VS. An endogenous stress hormone, vasopressin, also known as arginine vasopressin or ADH, is secreted from the posterior pituitary gland in response to decreased MAP, decreased plasma volume, and increased plasma osmolality.26 Vasopressin receptors (V1, V2, and V3) have the specific functions of vasoconstriction, osmoregulation via insertion of aquaporin channels in the kidney, and hypothalamic secretion of adrenocorticotropic hormone, the precursor to vasopressin, respectively.26 Vasopressin is most commonly administered as a continuous infusion because of its short plasma half-life of 4 to 20 minutes.26 A study reported in 2010 showed improved hemodynamics after coronary artery bypass grafting when a low-dose vasopressin infusion of 0.03 U/min was used during the perioperative period in patients with ejection fractions of 30% to 40% and who also continued taking their ACE inhibitors.9 Should VS develop after CPB, 0.01 to 0.1 U/ min of vasopressin can be given as a continuous infusion with minimal side effects.9 Unfortunately, literature regarding the use of intravenous bolus doses of vasopressin for the treatment of ACE inhibitor–associated refractory hypotension is limited. Several articles document the use of 1-mg intravenous bolus doses of terlipressin, a vasopressin agonist not available in the United States, as effective in www.aana.com/aanajournalonline treating intraoperative ACE inhibitor–associated refractory hypotension.4-7 The administration of terlipressin in combination with ephedrine has been shown to correct hypotension more rapidly than when either drug is administered alone.4 Similarly, bolus doses of terlipressin more rapidly corrected hypotension compared with an infusion of norepinephrine.7 Guidelines for treating ACE inhibitor–associated refractory hypotension with intravenous bolus doses of vasopressin agonist that are available in the United States are lacking. Side effects of vasopressin and vasopressin agonists are dose-dependent and include decreased organ perfusion, especially to the kidneys, liver, and mesentery due to increased SVR.6,26 Myocardial ischemia and cardiac arrest with higher doses can occur due to increases in SVR resulting in decreased cardiac output, decreased myocardial oxygen delivery, and increased myocardial oxygen demand.6,26 Methylene blue has been documented as effectively treating VS in the postoperative period and when given preoperatively for patients at high risk for VS.11-13 The use of methylene blue for treating ACE inhibitor–associated hypotension following the induction of anesthesia that is refractory to standard treatments has not been documented. The mechanism of action for methylene blue involves competition with NO for binding sites on guanylate cyclase.13 When methylene blue binds to guanylate cyclase, cyclic guanosine monophosphate levels do not increase and vascular smooth muscle vasodilation is inhibited.13 Methylene blue can be given as an intravenous infusion of 2 mg/kg infused over 20 minutes.13 It has been documented that this dose has reversed VS in approximately 2 hours.13 Research is limited on the use of methylene blue as a continuous infusion. Like vasopressin, side effects of methylene blue are dose-dependent. Transient arrhythmias and angina can occur, as can decreased cardiac output, decreased perfusion to the kidneys and mesentery, and increased pulmonary vascular resistance.13 Discoloration of the urine and skin can occur but is usually self-limiting.13 The administration of methylene blue causes interference with the light emission necessary for accurate pulse oximetry readings,13 and falsely low readings will be observed. Use of methylene blue is contraindicated in patients with severe renal insufficiency and should be used cautiously in patients with a deficiency of glucose-6-phosphate dehydrogenase because of the risk of hemolytic anemia.13 Conclusion The number of patients with hypertension undergoing surgery continues to increase. Patients are often prescribed 1 or more antihypertensive medications; therefore, anesthesia providers must be aware of the interactions between anesthesia and specific drug classes of antihypertensives. Unlike other classes of antihypertensive drugs, an unequivocal guideline for continuing AANA Journal April 2013 Vol. 81, No. 2 137 Figure 2. Postinduction ACE Inhibitor–Associated Refractory Hypotension Treatment Algorithm Abbreviation: ACE, angiotensin-converting enzyme. a Insufficient evidence to provide specific dosing guidelines for use of vasopressin or methylene blue for the use of ACE inhibitor– associated postinduction refractory hypotension. Figure 3. VS Treatment Algorithm Abbreviations: ACE, angiotensin-converting enzyme; VS, vasoplegic syndrome; CPB, cardiopulmonary bypass. a Insufficient evidence to support the use of intravenous bolus doses of vasopressin for treatment of VS. 138 AANA Journal April 2013 Vol. 81, No. 2 www.aana.com/aanajournalonline or withholding ACE inhibitors in the preoperative period has not been established, contributing to a potential lack of consistency and uncertainty in clinical management. The continued use of ACE inhibitors within 8 to 24 hours of general anesthesia can result in 2 types of hypotension: (1) intraoperative hypotension following the induction of anesthesia or (2) a more profound and persistent hypotension in surgical patients undergoing CPB. These 2 types of hypotension can be refractory to standard treatments. A continuous infusion of vasopressin has been used successfully in the treatment of VS, and intravenous bolus doses of terlipressin have been used successfully in treating intraoperative refractory hypotension following induction. Terlipressin is not available in the United States, and comparative doses of available vasopressin agonists for this purpose have not yet been documented. Methylene blue has been used successfully in the treatment of VS in the postoperative period and preoperatively for patients at high risk for developing VS. The literature is lacking on the use of methylene blue for the treatment of intraoperative ACE inhibitor–associated refractory hypotension following induction. Guidelines have not been established for vasopressin and methylene blue as treatment options for either type of ACE inhibitor–associated refractory hypotension, although several studies show their effectiveness. Without guidelines, managing ACE inhibitor–associated refractory hypotension is also lacking consistency and contributing to uncertainty in clinical practice. To promote consistency and evidence-based practice, a potential treatment algorithm for managing ACE inhibitor–associated refractory hypotension is proposed based on the current literature (Figures 2 and 3). 7. Boccara G, Ouattara A, Godet G, et al. Terlipressin versus norepinephrine to correct refractory arterial hypotension after general anesthesia in patients chronically treated with renin-angiotensin system inhibitors. Anesthesiology. 2003;98(6):1338-1344. doi:10.1097/00000542200306000-00007. REFERENCES 18. Stoelting RK, Hillier SC. Handbook of Pharmacology and Physiology in Anesthetic Practice. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:345-358. 1.Elisha S. Cardiovascular anatomy, physiology, pathophysiology, and anesthesia management. In: Nagelhout JJ, Plaus KK, eds. Nurse Anesthesia. 4th ed. St Louis, MO: Saunders Elsevier; 2010:465-503. 2. Holecki M, Szewieczek J, Chudek J. Effects of angiotensin-converting enzyme inhibitors beyond lowering blood pressure: are they important for doctors? Pharmacol Rep. 2011;63(3):740-751. 3. Comfere T, Sprung J, Kumar MM, et al. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100(3):636-644. doi:10.1213/01.ANE.0000146521.68059.A1. 4. Meersschaert K, Brun L, Gourdin M, et al. Terlipressin-ephedrine versus ephedrine to treat hypotension at the induction of anesthesia in patients chronically treated with angiotensin converting-enzyme inhibitors: a prospective, randomized, double-blinded, crossover study. Anesth Analg. 2002;94(4):835-840. doi:10.1097/00000539200204000-00011. 5. Eyraud D, Brabant S, Nathalie D, et al. Treatment of intraoperative refractory hypotension with terlipressin in patients chronically treated with an antagonist of the renin-angiotensin system. Anesth Analg. 1999;88(5):980-984. 6. Morelli A, Tritapepe L, Rocco M, et al. Terlipressin versus norepinephrine to counteract anesthesia-induced hypotension in patients treated with renin-angiotensin system inhibitors: effects on systemic and regional hemodynamics. Anesthesiology. 2005;102(1):12-19. doi:10.1097/00000542-200501000-00006. www.aana.com/aanajournalonline 8.Oh YJ, Lee JH, Nam SB, Shim JK, Song JH, Kwak YL. Effects of chronic angiotensin II receptor antagonist and angiotensinconverting enzyme inhibitor treatments on neurohormonal levels and haemodynamics during cardiopulmonary bypass. Br J Anaesth. 2006;97(6):792-798. doi:10.1093/bja/ael268. 9. Papadopoulos G, Sintou E, Siminelakis S, Koletsis E, Baikoussis NG, Apostolakis E. Perioperative infusion of low-dose of vasopressin for prevention and management of vasodilatory vasoplegic syndrome in patients undergoing coronary artery bypass grafting: a doubleblind randomized study [published online March 28, 2010]. Eur J Cardiothorac Surg. 2010;5(17):17. doi:10.1186/1749-8090-5-17. 10. Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588-595. doi:10.1056/NEJMra002709. 11. Leite EG, Ronald A, Rodrigues AJ, Evora PRB. Is methylene blue of benefit in treating adult patients who develop catecholamine-resistant vasoplegic syndrome during cardiac surgery? Interact Cardiovasc Thorac Surg. 2006;5(6):774-778. doi:10.1510/icvts.2006.134726. 12.Evora PRB, Ribeiro PJ, Vicente WV, et al. Methylene blue for vasoplegic syndrome treatment in heart surgery: fifteen years of questions, answers, doubts and certainties. Rev Bras Cir Cardiovasc. 2009;24(3):279-288. doi:10.1590/S0102-76382009000400005. 13. Shanmugam G. Vasoplegic syndrome: the role of methylene blue. Eur J Cardiothorac Surg. 2005;28(5):705-710. doi:10.1016/j.ejcts.2005.07.011. 14. Crystal GJ, Salem MR. The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg. 2012;114(3):520-532. doi:10.1213/ANE.0b013e3182312e21. 15. Brashers VL, McCance KL. Structure and function of the cardiovascular and lymphatic systems. In: McCance KL, Huether SE, Brashers VL, Rote, NS, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010:1091-1141. 16.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl S-b):S9-S18. 17. Cheung BMY. Blockade of the renin-angiotensin system. Hong Kong Med J. 2002;8(3):185-191. 19. Omboni S, Borghi C. Zofenopril and incidence of cough: a review of published and unpublished data. Ther Clin Risk Manag. 2011;7:459-471. 20. Colson P, Ryckwaert F, Coriat P. Renin angiotensin system antagonists and anesthesia. Anesth Analg. 1999;89(5):1143-1155. doi:10.1213/00 000539-199911000-00012. 21. Turan A, You J, Shiba A, Kurz A, Saager L, Sessler DI. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114(3):552-560. doi:10.1213/ANE.0b013e318241f6af. 22. Kheterpal S, Khodaparast O, Shanks A, O’Reilly M, Tremper KK. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(2):180-186. doi:10.1053/j. jvca.2007.12.020. 23. Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) [published corrections appear in Circulation. 2008;117(5):e154 and Circulation. 2008;118(9):e143- AANA Journal April 2013 Vol. 81, No. 2 139 144]. Circulation. 2007;116(17):e418-e500. doi:10.1161/CIRCULATIONAHA.107.185699. 24. Vieira da Costa V, Caldas AC, Nunes LGN, Beraldo PSS, Saraiva RA. Influence of angiotensin-converting enzyme inhibitors on hypotension after anesthetic induction: is the preoperative discontinuation of this drug necessary? Rev Bras Anestesiol. 2009;59(6):704-715. 25. Pigott DW, Nagle C, Allman K, Westaby S, Evans RD. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83(5):715-720. doi:10.1093/bja/83.5.715. 26. Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105(3):599-612, 639-640. doi:10. 1097/00000542-200609000-00026. 140 AANA Journal April 2013 Vol. 81, No. 2 AUTHOR Andrea Thoma, CRNA, MS, is a staff nurse anesthetist at Loyola University Medical Center, Maywood, Illinois. At the time this article was written, she was a student at Rosalind Franklin University of Medicine and Science, Nurse Anesthesia Program, North Chicago, Illinois. Email: [email protected]. ACKNOWLEDGMENTS I thank Susan McMullan, CRNA, MSN, for being a mentor during my work on this project. A special thank you to Jennifer Garcia Wojtczak, MD, for helping develop my interest in the topic of this article. To Sandra L. Larson, CRNA, PhD, APN, thank you for your ongoing support and encouragement during my anesthesia training. www.aana.com/aanajournalonline