* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Morphology of Thalamocortical Neurons Projecting
Bird vocalization wikipedia , lookup
Dendritic spine wikipedia , lookup
Subventricular zone wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neuroplasticity wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Environmental enrichment wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Convolutional neural network wikipedia , lookup
Biological neuron model wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Electrophysiology wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Single-unit recording wikipedia , lookup
Synaptogenesis wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Metastability in the brain wikipedia , lookup
Axon guidance wikipedia , lookup
Neural oscillation wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Multielectrode array wikipedia , lookup
Chemical synapse wikipedia , lookup
Hypothalamus wikipedia , lookup
Neural coding wikipedia , lookup
Mirror neuron wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Apical dendrite wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Nervous system network models wikipedia , lookup
Circumventricular organs wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroanatomy wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Optogenetics wikipedia , lookup
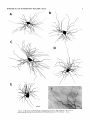
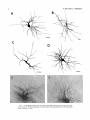
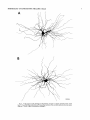
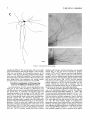
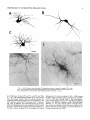
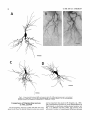
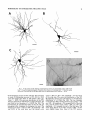
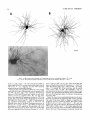
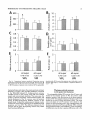
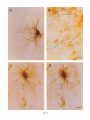
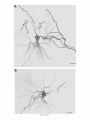
THE JOURNAL OF COMPARATIVE NEUROLOGY 361:1-24 (1995) Morphology of Thalamocortical Neurons Projecting to the Primary Somatosensory Cortex and Their Relationship to Spinothalamic Terminals in the Squirrel Monkey TING SHI AND A. VANIA APKARIAN Department of Neurosurgery, Computational Neuroscience Program, State University of New York Health Science Center, Syracuse, New York 13210 ABSTRACT This study examined the morphology of thalamocortical neurons projecting to the primary somatosensory cortex (SI; hand region of areas 3a, 3b, 1, and 2) and their relationship to the spinothalamic (STT) terminals in the squirrel monkey. Retrogradely labeled thalamocortical neurons were intracellularly filled with Lucifer yellow (LY), and the STT terminals were anterogradely labeled with biotinylated dextran. Both filled neurons and labeled terminals were differentially visualized in the same field by a dual immunocytochemical staining method. SI-projecting neurons appeared at the light level to be in contact with STT terminal boutons in the ventroposterior lateral (VPL), ventroposterior inferior (VPI), and centrolateral (CL) nuclei and the posterior complex (PO).The analyses of the neuronal morphology revealed that somatic and dendritic morphologies of SI-projecting neurons in these thalamic nuclei, as well as in the anterior pulvinlar (Pulo), centromedial (CM), and ventrolateral (VL) nuclei, were generally comparable with some exceptions: VL neurons had the largest soma sizes, the most primary dendrites, and the longest total dendritic length among all neurons studied; VPI neurons had the smallest soma sizes; VPL SI-projecting neurons were different from those in VPI in their soma sizes, shape factors, and orientations; in VPL the cells projecting to the superficial layers of SI were smaller than those projecting to the deeper layers, but in VPI the two groups of neurons were similar in soma sizes. In general, the SI-projecting neurons in VPL, VPI, and CL were similar in their dendritic morphologies and branching patterns, and varied from those in P u b , PO, CM, and VL. D 1995 WiIey-Liss, Inc. Indexing terms: intracellular injection, retrograde, anterograde, biotinylated dextran, fractal dimension The primary somatosensory cortex (SI) receives thalamocortical afferents from many thalamic nuclei. Its main input is from the ventroposterior lateral (VPL) and ventroposterior medial nuclei (VPM: for review, see Jones, 1985). A smaller number of cells projecting to SI are also found in the ventroposterior inferior (VPI), centrolateral (CL), and anterior pulvinar (Pulo) nuclei as well as in other thalamic nuclei (Jones and Leavitt, 1974; Jones et al., 1979; Friedman and Jones, 1981; Pons and &as, 1985; Cusick and Gould, 1990; Gingold et al., 1991). The neuronal morphologies in VPL or VPM (Yen and Jones, 1983; Yen et al., 1985; Harris, 1986; Chiaia et al., 1991; Nomura et al., 1992; Havton and Ohara, 1993, 1994; Ohara and Havton, 19941, CL (Yamamoto et al., 1985a; Tombol et al., 19901, the centromedial nucleus (CM, Hazlett et al., 1976; Yamamoto o 1995 WILEY-LISS, INC. et al., 1985b; Fenelon et al., 19941, and the ventrolateral nucleus (VL, Yamamoto et al., 1984, 1985a; Kultas-Ilinsky and Ilinsky, 1991) in the cat, rat, or monkey have been studied by means of Golgi preparations or intracellular injections of physiologically recorded neurons. Golgi preparations stain numerous neurons, but particular afferent and efferent connections may not be determined with these preparations alone, and although intracellular injections in physiologically characterized neurons can produce detailed morphological information in neurons with verified inputs Accepted March 16,1995. Address reprint requests to A. Vania Apkarian, SUNY Health Science Center at Syracuse, Neurosurgery Research Laboratory, 3118 WSK Hall, 766 Irving Avenue, Syracuse, NY 13210. T.SHI AND A.V. APKARIAN 2 and outputs, only a limited number of neurons can be evaluated in each animal. This study is aimed at examining the morphology of SI-projecting neurons in different thalamic nuclei and the relationship of these neurons to spinothalamic (STT) terminals in the squirrel monkey. In order to stain all the dendrites of identified SI-projecting neurons and STT terminals, anterograde and retrograde tracing techniques were combined with intracellular Lucifer yellow (LY) injection in fixed tissue (Buhl and Lubke, 19881, and a dual immunocytochemical staining method was developed. With this approach, the complete dendritic and somatic morphologies of labeled thalamocortical neurons were evaluated, and their proximity to anterogradely labeled STT terminals was identified in the same tissue. The somata and dendrites of the SI-projecting neurons from various thalamic nuclei were quantitatively analyzed and compared between the nuclei, and their relationships with the spinothalamic (STT) terminals were studied. From the earliest studies of Golgi impregnated cells (e.g., Cajal1911; Guillery, 1966; Tombol, 1967,19691,it has been observed that dendritic shapes in any given nucleus (or region) are highly variable. Cell morphology, size, and packing density are the main parameters by which various regions or nuclei have been delineated in the central nervous system. However, the variability in dendritic shapes is large enough that one can postulate that the morphology of neurons projecting to a single target from multiple nuclei may be more homogeneous than the general differences observed between these nuclei. The latter would imply that the innervating target would determine the detailed neuronal dendritic branching pattern. Alternatively, the neuronal morphology within multiple nuclei may be more homogeneous owing to a sharing of common afferent input. These hypotheses were directly tested in this study, and both were shown to be partially correct. MATERIALS AND METHODS Extracellular tracer injections This study utilized five adult squirrel monkeys (500-700 kg, both sexes). Three of the monkeys were used in an earlier study (Shi et al., 1993) to describe the locations of the retrogradely labeled neurons and their overlap with STT inputs. The housing, care, and surgical procedures followed the institutional guidelines established by the Committee for the Humane Use of Animals. One day prior to surgery, the monkeys were treated with dexamethasone (0.25 mgikg, i.m., twice daily) to prevent edema. On the day of surgery, doses of dexamethasone and the antibiotic Rocephin (75 mgikg, i.m.) were injected, and each animal was initially anesthetized with Ketamine (30 mgikg, i.m.). The surgical level of anesthesia in one animal was maintained with a Nembutal drip (0-10 mg/kg/h, i.v.) and Ketamine (10 mgikgih, i.m.1, whereas the other animals were maintained with 0.5-1.5% halothane or isoflurane mixed with ?hO2and % NzO. The animals were intubated in order to insure a patent airway. Intravenous fluid replacement with 5% dextrose lactated Ringer's solution was given during the experiment. Body temperature, expired GOz concentration, oxygen saturation (using a pulse oximeter), and heart rate were monitored non-invasively and were maintained within physiological limits. The anesthetized animals were placed in a stereotactic frame, and a craniotomy was performed under sterile conditions. The body map in SI was physiologically determined and marked on an enlarged color photograph of the cortical surface around the central sulcus. On one hemisphere, a retrograde fluorescent tracer, 2% Diamidino Yellow (DY), was deposited on the surface of the SI hand region (including areas 3a, 3b, 1,and 2) by placing a piece of DY soaked filter paper on the cortex for 20-40 minutes (for details see Shi et al., 1993; and Gingold et al., 1991). On the other hemisphere, or in different animals (see Table l ) , green beads (GB), 2% fast blue (FBI, or 2% DY, was injected into all cortical layers of the equivalent region of SI with a Hamilton syringe. In four of these animals, the cervical spinal cord was exposed through a laminetomy and 3.5-5 p1 of 2% wheat germ agglutinin-horseradish peroxidase (WGAHRP, Sigma) or 3.5-5 pl of 10% BD (biotin-dextran; Vector) in normal saline was injected in the C5-Tl gray matter with a glass pipette (for injection details, see Shi et al., 1993). The spinal cord WGA-HRP injections were done at the time of the cortical injections. The spinal cord BD injections were made 4-6 weeks prior to the cortical injections. Following surgery the animals were administered antibiotics for 3 days, and the animals that exhibited pain symptoms during recovery were administered analgesics (Torbugesic 0.05 mgikg, i.m.1. Tissue processing and intracellular injections Following appropriate survival periods, the animals were overdosed with Nembutal and perfused transcardially with normal saline and 2.5% or 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2). In addition, 0.025-0.05% glutaraldehyde was added to the fixative for two of the animals. Then, the brain was removed from the skull and three to four series of coronal vibratome sections were obtained and collected in a 0.1 M phosphate buffer (PB). One series of the sections (150 pm in thickness) were stored in 0.1 M PB at 4°C and used for intracellular filling within 2 weeks. Retrogradely labeled thalamocortical cells were observed under an epifluorescent microscope (excitation filters 395415 nm wavelength for DY and GB, 330-380 nm wavelength for FBI. Under direct visualization, retrogradely labeled cells in each of the thalamic nuclei were picked for intracellular injection of luicifer yellow. A glass pipette pulled on a vertical pipette puller (David Kopf Instruments, model 700C) and filled with a 4% aqueous solution of LY (LY-CH,dilithium salt, 41H3620, Sigma) was mounted on a micromanipulator attached to the microscope. A coronal thalamic section was placed on a plain glass slide, covered with a piece of filter paper in which a hole was punched, and TABLE 1. Tracer Injections' ~ Side SI injection sites Tracers Left Right Hand, surface SI Hand, all layer SI DY GB 2 Left Right Hand, surface SI Hand, all laver SI DY GB 3 Left Right Hand, surface SI DY 4 Left Right Left Hand, all layer SI FB Hand. all laver SI DY Animal no. 1 5 Spinal iniection C5-T1,3 5 pl WGA-HRP C5-Tl. 5 WGA-HRP C5-T1, 3.5 61 BD C5-T1,5 pl BD 'S1. somatosensory cortex; DY, diamidino yellow; GB, green heads; FB, fast blue; WGA-HRP, wheat germ agglutinin-horse radish pexoxidos. MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS 3 wetted with 0.1 M PB. Under visual control and a long working-distance objective (50 x ), a retrogradely labeled cell was intracellularly filled by delivering a negative current to the LY solution in the pipette through a piece of silver wire (5-10 nA, 0.5-2 min, W-P Instruments Inc., see Maranto, 1982;Buhl and Lubke, 1988).When the dendrites of a cell were completely filled, another cell was chosen and filled. with the Tablecurve program (Jandel Scientific). With the Tablecurve program, the distribution histograms of the soma sizes (the soma size versus the number of neurons) were fitted to normal distributions (one or two Gaussians). Staining of LY-filled neurons and BD-labeled STT terminals The extent of the cortical and spinal cord injections in three of the five animals is described in detail in an earlier study (Shi et al., 1993). The cortical and spinal cord injections in the other two animals were similar to the rest, i.e., the cortical injections were concentrated in the hand region of areas 3a, 3b, 1, and 2, whereas the spinal cord injections were mainly located in the gray matter of the cervical enlargement. The number of retrogradely labeled cells from the superficial SI were much fewer than those retrogradely labeled from all layer-SI, but their distributions in the thalamus were quite similar. Most retrogradely iabeied thalamocortical neurons from the SI hand region were primarily located in VPL, VPI, Pulo, and CL (for more details, see Shi et al., 1993). A small number of labeled neurons were seen in the posterior complex (PO), CM, and VL. Nearly 300 neurons whose dendrites were completely, or almost completely, filled were used in the dendritic analysis, which were located in VPL (n = 761, VPI (n = 651, Pulo (n = 611, PO (n = 30), CL (n = 411, CM (n = 121, and VL (n = 12). Figure 1 shows samples of coronal sections with LY-filled, immunocytochemically stained SI-projecting neurons in VPI. LY-filled cells were either photoconverted (see Maranto, 1982; Buhl and Lubke, 1988) or immunocytochemically reacted. Based on immunocytochemical methods to stain LY-filled cells (Brandon, 1985; Brandon and Criswell, 1991) and for BD (Brandt and Apkarian, 1992), a dual immunocytochemical staining method was developed. A section containing LY-filled cells was first placed in phosphate-buffered saline containing 0.1% Triton-X 100 and 1% normal goat serum (PBS-TX-NGS) for 20 mintues and then incubated in PBS-TX-NGS containing the Fab fragment of anti-LY antibody raised from rabbit (Fab-HCLY, 1:250, a gift from Dr. C. Brandon) overnight at room temperature. After washing the section twice in PBS-TX (0.5% Triton-X 100) for 10 minutes, the section was immersed in avidin-biotin complex (ABC-Kit, Vector) in PBS-TX solution for 2 hours at 37°C. After two washes in PBS-TX for 10 minutes, the section was reacted with 0.015% 3,5-diamino benzidine (DAB)in Tris-buffer (pH 7.6) containing 0.4% nickel ammonium sulfate and 0.005% H202for 10 minutes. This procedure stained the BD labeled terminals in a blue-black color. Following rinses in PBS, the tissue was incubated in PBS-TX-NGS containing goat antiserum against FabHCLY (150, Cappel) for 2 hours, and then in PBS-TX-NGS containing PAP (a complex of HRP and rabbit Fab fragment of antiserum to HRP, 1:100, Jackson Immunoresearch Laboratories, Inc.) for another 2 hours at 37°C. After each antibody incubation and the PAP reaction, the tissue was washed twice in PBS-TX-NGS for 10 minutes. The section was then incubated in 0.05%DAB in Tris buffer (pH 7.6) with 0.005% Hz02for 15 minutes at room temperature, rinsed in 0.1 M PB, and mounted out of 0.033M PB. All of the LY-filled cells in a section were stained brown, which was easily differentiated from the previously stained, blue-black STT terminals (see Fig. 16). RESULTS Soma morphology of SI-projectingneurons in different thalamic nuclei In VPL, SI-projecting neurons usually had medium-sized or larger somata with multipolar shapes and four to eight primary dendrites. Samples of LY-filled,immunocytochemically stained SI-projecting neurons located in VPL are shown in Figure 2. Most SI-projecting neurons in VPI were medium-sized or small, and had four to eight primary dendrites (see Fig. 3). The labeled SI-projecting neurons in Pulo were medium-sized to large, multipolar, and usually had five to nine primary dendrites with many branches (see Fig. 4). labeled SI-projecting neurons in PO were mediumsized or large, multipolar or round, and had three to seven primary dendrites (see Fig. 5 ) . The somata of SI-projecting Data collection and analysis neurons in CL were large or medium-sized, multipolar or The locations of the LY-filled SI-projecting neurons and triangular-shaped, and had three to eight primary denlabeled STT terminals were determined relative to thalamic drites with branched dend itic trees (see Fig. 6). The nuclear boundaries which were based on the background SI-projecting CM neurons usually had medium-sized, mulDAB staining and adjacent sections stained with cresyl tipolar somata and three to seven primary dendrites (Fig. violet or cytochrome oxidase (for details regarding determi- 7). The SI-projecting VL neurons were large in their soma nation of nuclear boundaries, see Gingold et al., 1991; Shi et sizes and had eight to 13primary dendrites (see Fig. 8).The al., 1993; Apkarian and Shi 1994). Samples of these filled locations of the neurons shown in Figures 2-8 are illuscells and STT terminals were drawn with a camera lucida trated in a schematic diagram of coronal sections of the and a computerized microscope system (Eutectic Electron- thalamus (Fig. 9). Samples of stained SI-projecting neurons are shown in ics, Raleigh, NC). The soma areas, soma shape factor, soma widths and heights, and the approximate dendritic fields Figures 2-8. In general, the soma areas of the retrogradely were measured by a computerized video system (Bioquant, labeled SI-projecting neurons varied from 78 pm2 to 752 R. M. Bioquantries). The shape factor = 4*a*areal km2 (mean 2 SD 325 ? 110, n = 262). Table 2 is a sumperimeter squared. The fractal dimension of the camera mary of the soma sizes and other measures of the SIlucida drawn neurons were measured using the box- projecting neurons in VPL, VPI, Pulo, PO, CL, CM, and VL. counting method (Krauss et al., 1994). The total dendritic The soma areas of the SI-projecting neurons in VPL length was measured with a computer program Mocha (351 & 135 pm2), Pulo (353 2 79 pm2),and CL (351 2 89 (Jandel Scientific). Statistical analyses were done with pm2) were slightly larger than those in PO (333 2 63 pm2), Sigmastat (Jandel Scientific), and curve fitting was done CM (320 2 60 km2), and VPI (249 ? 91 pm2). The SI- 4 T. SHI AND A.V. APKARIAN Fig. 1. A photomicrograph of Lucifer yellow (LY)-filled,immunocytochemically somatosensory cortex stained (SI) projecting neurons in ventro posterior inferior nucleus (WI) region of the left thalamus. Spinothalamic fibers coursing along the ventral border of VPI and within WI and ventroposterior lateral nucleus (VPL) can also he ob- served. The dashed line shows the border between VPI and dorsally adjacent VPL and ventroposterior medial nucleus (VPM). Scale bar = 50 km. Insert is the plot of this thalamic section indicating the location of the photomicrograph. Dorsal is up and lateral is left. The orientations of all other figures are the same, except Figure 17C, D. projecting neurons in VL had the largest soma areas (410 ? 142 pm2) among all of the LY-filled SI-projecting neurons in these thalamic nuclei. The comparison of the soma sizes between the different thalamic nuclei resulted in a significant difference (Kruskal-Wallis one-way ANOVA, P c .0001, Fig. 10A). Post-hoc pairwise multiple comparison (Dunn’s method) shows that VPI soma sizes are significantly smaller than VPL, Pulo, PO, CL, and VL ( P < ,051.All other pairs were not significantly different. The shapes of these SI-projecting neurons were multipolar, triangular, round, and flat. The average shape factor was highest for the VL cells (0.742 ? 0.051) and lowest for VPI cells (0.567 0.123, Fig. IOB).There was a significant difference in soma shape factor between the thalamic nuclei (Kruskal-Wallis one-way ANOVA, P < .0002). Post-hoc pairwise multiple comparison (Dunn’s method) shows that VL shape factors are significantly larger than VPI, VPL, Pulo, PO, and CL ( P < .05). All other pairs were not * MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS E Fig. 2. A-E: Camera lucida drawings of SI-projecting neurons in VPL. Scale bar = 30 pm. F: A photomicrograph showing the smooth dendrites of SI-projectingneuron in W L . Scale bar = 10 km. 5 T.SHI AND A.V. APKARIAN 6 A D \- Fig. 3. Camera lucida drawings (A,D) and computer plots (B,C) of SI-projectingneurons in VPI. Scale bars = 30 km. E, F: Photomicrographs of typical dendritic branching patterns for WI SI-projecting neurons. Scale bar = 10 km. MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS i B / 7 i \ Fig. 4. A-C: Camera lucida drawings of SI-projecting neurons in anterior puloinlar (Pulo). Scale bars = 30 pm. D,E: Typical dendrites of 51-projectingneurons in Pulo on which there are a few tiny spines. Scale bar = 10 pm. (Figure 4 continued on next page.) 8 T. SHI AND A.V. APJSARIAN Figure 4 (Continued from previous page.) significantly different. The average ratio of the soma width to the soma height in VPI was the largest (1.183 ? 0.409). This ratio was smallest for SI-projecting neurons in CL (0.894& 0.275, Fig. 1OC).However, these orientation differences were not statistically significant. These measures for the SI-projecting neuronal somata indicate that the soma area, shape factor, and orientation are usually similar across the nuclei, with the exceptions of VL and W I . Dendritic morphology of SI-projecting neurons in different thalamic nuclei In coronal sections, the VPL neurons had dendritic trees with two to four order branches in dichotomous and tuft-like patterns, that covered all possible directions from the somata (Fig. 2). Generally, the dendritic sizes and branching patterns of the neurons in VPL and VPI were similar (Fig. 3), except for the VPI neurons located on or near the ventrolateral border which rarely had dendrites directed outward (Fig. 3C). Dendritic appendages, e.g., dendritic swellings, beads, or spines, were only occasionally found on VPL and VPI dendrites (Figs. 2, 3). The primary dendrites of Pulo neurons were usually short and richly branching. Dendritic appendages were rarely seen on the Pulo dendrites (Fig. 4).A few Pulo neurons had a small number of primary dendrites with long dendritic branches (Fig. 4D). The PO neurons usually had fewer primary dendrites and branches, and their dendrites were basically smooth (Fig. 5). The CL neurons branched in patterns similar to W L and VPI neurons, and some had dendritic swellings (Fig. 6 ) . The primary dendrites of CM neurons seemed to be of two different types: sparsely branching and densely branching. The densely branched dendrites often had curves and spines (Fig. 7A,C,D) and the sparsely branched ones were straight and smooth (Fig. 7B). Some primary dendrites in the VL neurons branched near the somata, whereas the others had no branches. The VL dendrites were basically smooth (Fig. 8). In the majority of the neurons the axons were not readily identifiable. The numbers of primary dendrites of SI-projecting neurons were significantly different between the different thalamic nuclei (Kruskal-Wallis ANOVA, P < .0001). The VL neurons had the most primary dendrites (10.16 ? 1.751, and the CM neurons had the least (4.483? 1.19, Fig. 11A). Post-hoc pairwise multiple comparison (Dunn's method) shows that the numbers of primary dendrites for VL and Pulo were different from the neurons in the other nuclei ( P < .05). The total dendritic length for individual SIprojecting neurons were measured from two-dimensional drawings. The dendritic lengths of SI-projecting neurons were significantly different between the different thalamic nuclei (Kruskal-Wallis ANOVA, P < .05). Post-hoc pairwise multiple comparison (Dunn's method) shows signifi- MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS 9 C Fig. 5. A-C: Camera lucida drawings of SI-projecting neurons in posterior complex (PO). Scale bar = 30 Fm. D, E: Photomicrographs of SI-projecting neuron and dendrites in PO. Dendritic spines can be seen in D. Structures out of focus are mainly spinothalamic fibers. Scale bar = 10 Fm. cant differences between PO and VL, and PO and Pulo ( P i.05). The total dendritic lengths of the VL neurons were the longest (4,684 ? 582 pm) among all-layer SIprojectingneurons, followed by Pulo neurons (3,699 2 1,865 pm), and were shortest for PO neurons (1,251 2 388 pm, Fig. 11B). In addition, the fractal dimensions of the dendrites of individual neurons were measured, and the result of such a measurement using box-counting method for a VPI cell is shown in Figure 12A. On average, the fractal dimensions in VL were the highest (1,410 2 0.050) among all these nuclei, whereas those in PO were the smallest (1,273 0.115, Fig. 12B and Table 2). The fractal dimensions of SI-projecting neurons were significantly different between the different thalamic nuclei (Kruskal-Wallis ANOVA, P < .05). The relative pattern of fractal dimension across the thalamic nuclei was very similar to the pattern observed for the number of primary dendrites in the same nuclei (see Fig. 12A,B). * T. SHI AND A.V. AF’KARIAN 10 A \ - Fig. 6 . Camera lucida drawings (A,D) and computer plots (C) of SI-projecting neurons in centrolateral nucleus (CL). Scale bars = 30 pm. B: A photomicrograph of dendrites shown in A. The corresponding dendritic branches are indicated in A and B with “v.” Scale bar = 10 am. Comparisons of SI-projectingneurons in VPL and VPI The SI-projecting neurons in VPL and VPI were compared, since these two lateral thalamic nuclei were consid- ered as important relay nuclei to SI (Gingold et al., 1991). The cytoarchitecture of these two nuclei looked different in Nissl-stained or cytochrome oxidase-stained sections (ref. Fig. 1 in Apkarian and Shi, 1994). The present study uncovered a number of morphological differences between MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS 11 B Fig. 7. A-C: Camera lucida drawings of SI-projecting neurons in centromedial nucleus (CM). Scale bars = 30 Fm. D: A photomicrograph of dendrites from neuron in C, indxated with “v.” Dendritic spines can be seen. The thick fiber on the upper right corner is a spinothalamic axon. Scale bar = 10 Fm. the SI-projecting neurons in VPL and VPI. The soma sizes or areas of SI-projecting neurons in VPL (351.5 t 135.2 pm2) were larger than those in VPI (249.5 t 90.8 pm2, t-test P < .0001). The soma size distribution of the VPL neurons could not be well fitted by a single Gaussian, but was fitted by two Gaussians (r2= 0.9139, Fig. 13A). The two-Gaussian fit remained the better fit for the cell size histograms after changing the histogram bin size. The sharper curve (mean = 335 pm2,SD = 28, amplitude = 26) was 23.3% of the total area, and the second curve (mean = 389 pm2, SD = 158, amplitude = 15) was 76.7% of the total area. The soma size distribution of the VPI SI-projecting neurons was also fitted to a two-Gaussian distribution (r2= 0.7269, Fig. 13B). The two Gaussian curves had very little overlap. The first curve (mean = 273 pm2, SD = 90, amplitude = 20) occupied 96.8% of the total area, and the second curve (mean = 565 pm2, SD = 28, amplitude = 2.1) occupied the rest of the total area. The soma shape factors of the VPL neurons (0.607 2 0.107) were slightly larger than those of the VPI neurons T. SHI AND A.V. APKARIAN 12 B Fig. 8. A, B: Camera luicda drawings of SI-projecting neurons in ventrolateral nucleus (VL). Scale bar = 30 km. C: A photomicrograph of another VL neuron with smooth dendrites. Scale bar = 10 pm. (0.567 t 0.123, t-test P < .05). The ratios of soma width to soma height of the VPL neurons were smaller than those of VPI (t-test P < . O l ) , implying that the VPI neuronal somata are more horizontally oriented. Although there was a significant difference in the average soma sizes of all-layer %projecting neurons between VPL and VPI, the soma sizes of the superficial SI-projecting neurons in VPL and VPI were very similar to each other (249.41 5 102.16 vs. 239.59 t 97.01, Fig. 14). The soma sizes of the superficial SI-projecting neurons in VPL were smaller than those of the all-layer SI-projecting neurons in VPL (402.13 t 125.25, t-test P < .0001). The soma sizes of the superficial SI-projecting neurons in VPI were not different from those of all-layer SI-projecting neurons in VPI (271.75 t 72.26, t-test P > .05). When the soma size distributions of the superficial SI-projecting cells were fitted with Gaussian curves, these soma sizes of the VPL cells were adequately fitted with one Gaussian (r2= 0.975, mean = 245 pm2,SD = 61, Fig. 13C). Those of the VPI cells were still better fitted with two Gaussians (r2= 0.98, Fig. 13D). The sharper peak (mean = 205 p,m2, SD = 25, amplitude = 7.1) covered 32% of the total area, and the second curve (mean = 245 pm2, SD = 82, amplitude = 4.6) covered the rest of the area. The relative contributions of each curve for the superficial-SI projecting neurons seem different from that described above for all-layer SI-projecting neurons in VPI. Figure 14 shows other morphological properties of superficial SI and all-layer SI-projecting cells in VPL and VPI. The ratio of the soma width to height of superficial SIprojecting neurons in VPI was higher than that in VPL (1.228 2 0.44 vs. 1.010 & 0.32, t-test P < .05) and also higher than that of the all-layer SI-projecting neurons in VPI (1.080 & 0.300, t test P < ,051, which means that this group of neurons was more horizontally oriented than the others. The rest of the parameters measured showed that MORPHOLOGY OF Sl-I'ROJECTING THALAMIC CEI,I,S AP 4.6-5.0 13 5.3-5.6 6.2-7.4 Fig. 9. A schematic diagram of coronal thabdrnic sections a t difkrent anterior-posterior levels. 'I'hc locations of the %projecting neurons in each of these thalamic nuclci, shown in Figures 2 -8, are shown with t h e same alphabets a s used in Figures 2-8. TAULE 2. Summary of Soma a n d I l e n d r i t i c M e a s u r e s i n Each Thalamic N ucleus' VPL VPI Number of neurons Soma area (krn2) Soma shape factor Ratio of soma x l y Number of primary dendrites Total dendritic length (pmV 76 351 i 135 0.607 0.107 1.013 2 0.266 6.21 i 1411 2,976 e 770 l+:ict,:d dirncnswri2 I . X l I0.49 n = 19 65 249 ? 9 1 0.567 ? 0.123 1.183 t 0.49 6.40 ? 1.60 2,864 t 1,093 n = 13 1.352 i 0.081 n = 11 * = m Pulo the dendrites of the superficial SI-projecting cells were similar to those of all-layer SI-projecting cells in the two nuclei. Nine SI-projecting neurons in VPL (total of 41 dendritic trees) and five SI-projecting neurons in VPI (total of 32 dendritic trees) were analyzed after tracing them with the computerized neuron tracing system. The stem diameters of the primary dendrities, the total dendritic length, the total dendritic membrane areas, and the numbers of the dendritic branch points for individual dendritic trees were similar between these two nuclei. There were strong positive correlations between the primary dendritic diameter and the total dendritic length (r = 0.584, P < .0001 in VPL; r = 0.599, P < .001 in VPI), the total dendritic area (r = 0.684, P < .0001 in VPL; r = 0.688, P < .0001 in VPI), and the number of the branch points (r = 0.559, P < .0001 in VPL; r = 0.654, P < ,0001 in VPI; see Fig. 15A-C). The slopes of diameter versus total length and diameter versus total area were not significantly different between VPL (370.6, 650.0) and VPI cells (272.7, 389.9, Fig. 13A, B). The slopes for diameter versus branch points for VPL (6.54) and VPI (3.27) were significantly different PO CL CM vr, 30 333 t 63 0.592 i 0.100 1.045 I(l.240 5.43 t 1.57 1,376 i 472 n = 3 1.273 i 0.115 n = 6 41 351 ? 89 0.572 -t 0.121 0 8514 I0 275 5.58 ? 1.51 2,350 i 757 n=9 1.352 t 0.027 n = 9 12 320 i 60 0.624 2 0.091 12 410 i 142 0.742 i 0.051 1 00.1 t 0 214 1 Ill7 4.48 i 1.19 3,127 i 1,126 n=4 1.283 ? 0.100 n=4 10.16 2 1.75 4,684 ? 582 n = 8 1.410 i 0.050 n = 8 6 0 20% from each other (t-test, P < .0001). There were also strong positive correlations between the numbers of the branch points and the total dendritic length in these two nuclei (r = 0.848, P < .0001 in VPL; r = 0.90, P < ,0001 in VPI; see Fig. 15D). The slope between the numbers of the branch points and the total dendritic length in VPL (45.7) was significantly less than that in VPI (82.0, t-test, P < .0001). Relationship between SI-projectingneurons and spinothalamic terminals The S'I'T terminals anterogradely labeled from the contralateral cervical enlargement were found in several thalamic nuclei, including VPL, VPI, PO, CL, and sparsely in Pulo as well as in the medial thalamic nuclei (see Gingold et al., 1991; Shi et al., 1993). Since the labeled STT terminals in the thalamus were a series of clusters, the approximate locations of STT terminals were determined before intracellular injections by examining the stained, BD-labeled STT terminals in the alternative serial sections. Figure 16A is a color photograph of a Pulo SI-projecting cell (brown), and Figure 16B shows a field in CL where STT terminals can be T.SHI AND A.V. AF'KARIAN 14 A 600 cn a, .-c a - € T T I .2 400 : g (d T -z AU l-l 1105 1 200 0 Lc cn 0 5tr 0 1 VPLVPI Pulo PO CL C M VL 0.8 c -$ 2.0 4 I T V P L V P I Pulo PO CL CM VL € (I) 0 1.0 0 .- 5 0.5 (r 0.0 1 m 2 v 6 ca, z 2 (d w 0 I VPLVPI Pulo PO CL CM VL T 0 L B-E 8 Fig. 11. A The numbers of the primary dendrites (A) and the total dendritic (den.) lengths (B) measured from individual neurons for SI-projecting neurons in the thalamic nuclei indicated. T 1.5 VPL VPIPulo PO CL C M VL I I I I I I 11 VPLVPI Pulo PO CL CM VL Fig. 10. The soma areas of the SI-projectingneurons in each of the thalamic nuclei indicated. B: The soma shape factors of the neurons. C : The ratio of soma width (x)to soma height (y). seen in blue-black, while the dendrites of two SI-projecting cells are shown in brown. In these sections, many STT terminal boutons were located between Nissl-stained somata, and only a few appeared to contact the somata, implying that most STT terminals synapse on the dendrites of thalamic neurons. A stereo-pair of color photos of a SI-projecting cell in C1 with STT terminals abutting one of its dendrites is shown in Figure 16C,D (three-dimensional microscope, Edge Scientific Instrument Gorp., Santa Monica, CAI. The same neuron and the STT terminals are shown in more detail in drawing in Figure 17A. A total of ten SI-projecting cells were observed in similar close proximity to, or seemingly directly contacting, STT terminals, of which, two cells were in VPL, four were in VPI, two were in CL, and two were in PO. Drawings of synapse-like STT boutons abutting SI-projecting cells and other nearby STT axons and terminals are shown for four neurons in Figure 17. All the synapse-like boutons were contacting the body of the primary, secondary, and tertiary dendrites. DISCUSSION Intracellular labeling of the entire neuron Neurons intracellularly filled with LY reveal neuronal morphology at the same degree of detail as Golgi-impregnated material, as a result the method is widely used (Buhl and Lubke, 1988; Buhl et al., 1989; Buhl, 1993). Many laboratories have tried intracellular injection of HRP or other tracers in thalamic neurons of live animals and have filled a few labeled neurons in the cat (Yen and Jones, 1983; Yamamoto et al., 1984; Yen et al., 1985; Nomura et al., 1992), rat (Ohara and Havton, 19941, or monkey (Havton and Ohara, 1993).Since the number of the neurons intracellularly injected in alive animals, especially in the monkey, is MORPHOLOGY OF SI-PROJECTING THALAMIC CELIAS A h 104, I I I i 103 1 15 missed, but this should not significantly influence the results of the present study. Thalamocortical neurons in the lateral thalamus In the lateral thalamus, thalamocortical neurons have been studied in the cat (Yen and Jones, 1983; Yen et al., W 1985; Penny et al., 19831, rat (Peschanski et al., 1984; U Harris, 1986; Chiaia et al., 1991; Ohara and Havton, 1994), a, and monkey (Havton and Ohara, 1993, 1994). Yen and 1o2 Jones (1983; Yen et al., 1985) intracellularly injected HRP v) into physiologically identified neurons in the cat ventrobasal complex, and divided these neurons into three classes: 0 type I, type 11, and type 111. The type I and I1 neurons are rn 10' relay neurons, and the type I11 neurons are interneurons. Many of our LY-filled neurons in VPL looked like their type I neurons, with medial or large somata, large and smooth primary dendrites radiating in all directions from the soma, 100 and tufts of multiple second-order dendrites. A small 1 10' 1o2 103 104 number of the LY-filled VPL thalamocortical neurons resembled type I1 neurons, with smaller soma sizes, thinner dichotomously branching primary dendrites, and with some Size of boxes dendritic appendages. The soma size distribution of the VPL SI-projecting cells was better fitted to a two-Gaussian 1.5 curve, which implies two populations of SI-projecting neurons in VPL. The population with large mean and standard n T W deviation is similar to cat type I neurons, and the popula[I: 1.4 tion with small mean and standard deviation is similar to 0 .cat type I1 neurons (compare Fig. 7 in Yen et al., 1985 to Fig. 14A). 1.3 In general, the dendritic branching pattern of the VPL SI-projecting neurons in the present study was similar to 5 1.2 the macaque VPL thalamocortical neurons intracellularly recorded and filled with HRP by Havton and Ohara (19931, a .c.' but the soma sizes and the total lengths of the dendrites of 1.1 our VPL neurons were smaller and shorter. The differences L should be due to the different species and also, perhaps, to 1 differences in methodology. In the monkey, VPI neurons project to both SII (FriedVPL VPI Pulo PO CL CM VL man and Murray, 1986; Stevens et al., 1993) and SI (Cusick Fig. 12. A The fractal dimension measurement of a SI-projecting and Gould, 1990; Gingold et al., 1991; Shi et al., 1993). Because little is known about the neuronal morphology in neuron in VPI. B: The fractal dimensions of the SI-projectingneurons in each of these thalamic nuclei. VPI, we explored many SI-projecting neurons in this nucleus. In comparison with VPL SI-projecting neurons, the VPI SI-projecting neurons generally have smaller soma sizes, more horizontally oriented somata, and similar denusually very low, intracellular tracer injection in the fixed dritic lengths and fractal dimensions. The soma size distritissue is much more convenient to systematically study a bution in both VPL and VPI neurons projecting to all layers great number of neurons in a small number of animals. For of SI was well fitted to a two-Gaussian curve. These fits example, as many as 40 neurons were filled in one fixed imply that the VPL and VPI neurons consist of two section in the present study. Although LY-labeled cells can populations. Some VPI neurons had relatively more denbe directly observed with an epifluorescent microscope, dritic appendages than most VPL neurons. The dendriticpermanent staining is necessary for long-term or electron rich domain of VPI neurons implies that VPI neurons are microscopic study. Both photoconversion and immunocyto- able to receive at least as much information as VPL neurons chemical staining methods were used in the present study. and play an important role in the processing of somatosenThe LY photoconversion method only stained a couple of sory inputs. Recently, we studied the physiological features LY-filled cells per section. In contrast, the immunocyto- of VPL and VPI neurons in the squirrel monkey (Apkarian chemical method (Brandon, 1985; Brandon and Criswell, and Shi, 1994).Although the proportions of the nociceptive 1991) is better in that all LY-filled cells in a section can be neurons in the two nuclei were different, the general stained at once. Only retrogradely labeled cells with somata physiologic features of VPI neurons were similar to those of estimated to be in the middle of the thickness of the section VPL neurons, i.e., both of them could be classified into were filled with LY. If such a cell was successfully injected, low-threshold, wide dynamic range, and high-threshold most dendrites of the cell appeared to be filled and stained. types. The morphological similarities between filled VPL The possibility cannot be excluded that a few of the and VPI neurons are consistent with the physiological dendrites toward the top or bottom of the section were findings. cc, n % oo 1 E - .o T. SHI AND A.V. APKARIAN 16 50 A .. 40 Y) f - 30 0 0 450 2 0 5 20 0 900 450 900 Sansarca(mioon2) Sana area (rniuon2) z la a 200 400 800 600 Soma size (um2) Soma size (um2) 15 12 Y) 0 = 9 I c A 0 9M) 450 0 450 L m 900 n 5 6 z 3 2A 0 0 200 Soma 400 size (um2) 600 800 Soma size (um2) Fig. 13. The curves of the soma size distributions of VPL (A,C) and W I neurons (B,D).A: All-layer SI-projecting neurons in W L . B: All-layer SI-projecting neurons in VPI. C: Superficial SI-projecting neurons in VPI. D: Superficial SI-projecting neurons in P I . The inserts show individual or multiple Gaussian fits. Within cat ventrobasal complex (Penny et al., 1983; Rausell and Avendaiio, 1985) and monkey VPL (Rausell et al., 1992), the neurons that project to the superficial layers of SI usually have smaller soma sizes than the neurons that project to the deeper layers. We retrogradely labeled thalamic neurons from the superficial layers or all layers of SI and compared the superficial SI-projecting neurons with all-layer SI-projecting neurons in VPL and VPI. The soma sizes between these two groups of neurons are different in VPL, but not in VPI. The superficial SI-projecting neurons in VPL were similar in soma size to both superficial and all-layer SI-projecting neurons in VPI. The soma size distribution of the superficial SI-projecting neurons in VPL was better fitted to a one-Gaussian curve, whereas the superficial SI-projecting neurons in VPI was better fitted to a two-Gaussian curve. It seems that only one population of neurons in VPL, the group with smaller mean and standard deviation in soma size, projects to the superficial layers of SI. Conversely, the curve fits for VPI cells imply the existence of three different populations of SI-projecting neurons: one group that projects to superficial and deep layers of SI, medium-sized with large variability; a second group that projects mainly to superficial SI, small-sized with small variability; and a third, largest-sized population that only projects to the deeper layers. In the present study and in previous studies (Gingold et al., 1991; Shi et al., 19931, tracer injections in SI of the squirrel monkey always resulted in some retrogradely labeled neurons located in caudal VL. We cannot exclude that the VL neurons were retrogradely labeled from the adjacent primary motor cortex area 4, owing to tracer spreading, since early studies (Friedman and Jones, 1981; see reviews in Jones, 1985) show that the cells in the caudal part of VL project to the primary motor cortex, but not to SI. In the cat, Yamamoto et al. (1985a) intracellularly recorded and stained neurons with HRP. A few of the neurons were thalamocortical relay neurons and the rest were thalamo-caudate relay, entopeduncular-responsive, and cerebellar-responsive neurons. The soma sizes of the VL neurons in the present study were smaller than those described in the cat, whereas the dendritic fields were similar. Some WGA-HRP retrogradely labeled VL neurons in the macaque monkey were studied a t light and electron microscopic levels (Kultas-Ilinsky, 1991). This retrograde MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS A 600 1 m m2! 400 m I 6 a 17 * T T U z 6 .-€ 4 - 200 T L “ 2 0 0 c 0.8 20 0.6 a, 0.4 m + 0- a T T T T 0 I cd m cn 0.2 I= 0.0 F mc I L $ cc 1 T T T i i a u 1 - 0 0 .-0 0 .+ m + m u 21 U o 0 all super all super all super n=46 n=32 n=20 n=45 n=46 n=32 VPL VPI VPL all super n=20 n=45 VPI Fig. 14. Comparisons between superficial SI-projecting neurons and all layer SI-projecting neurons in VPL and W I . A Soma areas. B: Soma shape factor. C : The ratio of the soma width (xJto soma height (y). D: The numbers of primary (prim.) dendrites. E: The approximate dendritic fields. F: The ratio of the dendritic (den.) field width (x)to the dendritic field height (y). Asterisk indicates a significant difference (t test,P < .05). tracing technique only stains the somata and some primary dendrites, as well as a few secondary dendrites. Compared to the WGA-HRP-labeled VL thalamocortical neurons, LY-filled neurons in the present study had more inflated somata and more primary dendrites. The soma sizes, the number of primary dendrites, total dendritic lengths, and fractal dimensions of the VL neurons were the greatest among all filled SI-projecting thalamic neurons in the present study. Unlike other somatosensory thalamic nuclei, VL does not receive direct inputs from the medial lemniscal projection, nor from STT. The difference between the VL neurons and the other SI-projecting neurons may reflect their functional difference. Thalamocortical neurons in the posterior thalamus The retrogradely labeled PO neurons from SI were only located in the anterior portion of PO, the portion called posterior nucleus. Only the neurons in the more posterior portion of PO (the supergeniculate and limitan nuclei) were studied in the cat by Winer and Morest (1983). The soma sizes and shapes of our PO neurons were similar to the somata of those principal cells in the suprageniculate and limitans nuclei shown by Winer and Morest (1983), whereas their dendritic domains were different. These morphological differences may be due to species differences and to the T. SHI AND A.V. APKARIAN 18 A 2000 1500 / / / / 1000 500 0 0 1 2 3 4 5 0 6 n cu E 2 3 4 5 6 Prim. Den. Diameter (pm) Prim. Den. Diameter (pm) C 1 D 2000 2400 . 1 I W m 1500 2? 1600 m a, C ! E 1000 4 2 m 800 500 + 0 t- 0 0 0 1 2 3 4 5 6 Prim. Den. Diameter (vm) 0 4 8 12 16 20 Branching points Fig. 15. The relationships between the primary (prim.) dendritic (den.) diameter and the dendritic length (A), the dendritic areas (B), and the number of the branching points (C) on individual dendritic trees of SI-projectingneurons in VPL (triangles and solid line) and VF'I (dots and dashed line). D: The relationships between the number of branching points and the total dendritic length. different subdivision where PO neurons were located. Alternatively, when compared with the SI-projecting neurons in VPL and VPI, PO SI-projecting neurons were similar in soma size and different in total dendritic length and dendritic fractal dimension. The differences in the dendrites is probably due to the fact that some PO dendrites had sparse branches. Earlier studies indicate that Pulo neurons can be retrogradely labeled from area 2 of SI (Pons and Kaas, 1985). Tracer injections in SI of squirrel monkeys result in a great Fig. 16. A: Photomicrographs of a Lucifer yellow (LY)-filled and immunocytochemically stained SI-projecting neuron in Pulo. B: The dendrites of a LY-filled and immunocytochemically stained SIprojecting neurons (orange brown) and nearby BD-labeled spinothalamic (STT) terminals (blue black) in CL. In A and B, dorsal is left. C, D: A pair of stereoscopic photomicrographs of a SI-projecting neurons (orange brown) contacted by STT terminal boutons (blue black) in centrolateral (CL).This pair of photomicrographswere taken through a three-dimensional microscope (Edge Scientific Instrument Corp.). In C and D, dorsal is up. Scale bars = 20 pm. Figure 16 Figure 17 Aand B MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS 21 Fig. 17. Drawings of SI-projecting neurons with contacting STT terminal boutons in W L (A),WI (B) and CL ( C ) .Arrows point to the contacts between the dendrites and the boutons. The left neuron in C is the same as in Figure 16C,D. Scale bars = 20 Fm. number of retrogradely labeled cells in Pulo (Gingold et al., 1991; Shi et al., 1993). In the present study, the LY-filled neurons in Pulo had soma sizes similar to those in VPL and PO. However, the Pulo neurons had more primary dendrites, longer total dendritic length, and slightly higher dendritic fractal dimension than the SI-projecting neurons in the other thalamic nuclei, except those in VL. The pattern of the dendritic distribution of some Pulo neurons is similar to VPL neurons, whereas the dendritic distribution of other Pulo neurons resembles VL SI-projecting neurons. As found in most other thalamic nuclei, the Pulo neurons also had few dendritic swellings. A comparison of neuronal morphologies between Pulo, PO, VPL, VPI revealed that the SI-projecting neurons in Pulo were quite different from those in the other thalamic nuclei in their dendritic domain, suggesting that SI-projecting neurons in Pulo may have a distinct physiologic function. Thalamocortical neurons in thalamic intralamina nuclei Anatomic studies have shown that CL is a major intralaminar nucleus which projects to SI (Jones and Leavitt, 1974; Jones et al., 1979; Bentivoglio et al., 1983; Gingold et al., 1991; Shi et al., 1993), whereas only a few CM neurons can be retrogradely labeled from SI (Bentivoglio et al., 1983; Gingold et al., 1991; Shi et al., 1993). CL and CM neurons can respond to noxious and other sensory stimuli (Dong et al., 1978; Dostrovsky and Guilbaud, 1990). Recently, Fenelon et al. (1994) have studied the neuronal morphology in the central complex (CM and Pf in our terminology) of both macaque monkey and human thalami by means of the Golgi method. These researchers classified the central complex into three subdivisions, and indicated that the large, principal neurons in the different subdivisions were different in their dendritic domains. The dendritic branch pattern and the dendritic shapes of our CM SI-projecting neurons are similar to those of the macaque neurons located in the most lateral subdivision, pars paralateralis, and in the medial subdivision, pars media. To date, the neuronal morphology of only a few CL neurons have been were explored in the cat (Yamamoto et al., 1985a; Tomb61 et al., 1990). We intracellularly labeled many SI-projecting neurons in CL, and compared them with other SI-projecting neurons. The measured parameters for somata and dendrites of the CL neurons were not significantly different from those parameters measured for the SI-projecting neurons in CM, VPL, VPI, and PO. However, the CM dendrites had many dendritic spines, but the CL dendrites did not, although both nuclei are in the intralaminar region. Surprisingly, the SI-projecting neurons in CL were more similar to those in VPL than those in CM in their soma sizes, dendritic branching patterns, and dendritic appendages. Since both CL and VPL receive medial lemniscal and STT inputs, the similarities between the SIprojecting neurons in the two nuclei implies that similar afferent inputs may somehow alter the dendritic morphology of their target neurons, and that the neurons in CL may 22 play an important role in processing somatosensory information. Relationship between SI-projectingthalamic neurons and STT terminals For many years it has been indicated that the STT inputs can be conveyed into SI (Jones, 1985; Kaas and Pons, 1988; Gingold et al., 1991; Shi et al., 1993; Apkarian and Shi, 1994) since STT projects to the thalamus and the thalamic neurons project to SI. Ralston and his coworkers (1985) studied STT terminals anterogradely labeled with HRP within VPL of the cat, rat, and monkey at the electron microscopic level. They showed that the STT terminals synapsed on the dendrites of VPL neurons that were regarded as thalamocortical because these neurons were not interneurons or GABAergic neurons (Ralston and Ralston, 1992). In order to anatomically explore STT connections with thalamocortical neurons whose cortical projections are identified, multiple tracing techniques are necessary. Buhl et al. (1989; Buhl, 1993) used an anterograde degeneration technique together with intracellular injection of LY in retrogradely labeled cortical neurons. Degeneration technique does not adequately trace the STT terminals in the primate. Recently, a technique of anterograde tracing with Phaseolus uulgaris-leucoagglutinin (PHA-L) and retrograde fluorescent tracing combined with intracellular injection of LY was developed in the rat by Wouterlood et al. (1990). PHA-L has been tried in our laboratory for labeling monkey STT terminals, and few or no terminals were well-labeled. Since BD can label both large and small fibers or terminals in the monkey (Brandt and Apkarian, 1992; Shi et al., 19931, we used BD to label STT terminals and LY to further label SI-projecting cells in the present study. We found that many STT boutons were near the filled SI-projecting cells and their dendrites. However, only a few STT boutons seemed to contact the dendrites of the intracellularly labeled cells. This is the first study to show STT terminals contacting the identified SI-projecting thalamic neurons in the primate at the light microscopic level. This finding is consistent with the electron microscopic study by Ralston et al. (1985) and further supports the idea that STT inputs are able to be directly relayed to SI not only through VPL but also through VPI, CL, and PO. Compared with earlier studies (Gingold et al., 1991; Shi et al., 1993), the percentage (less than 5%) of the SIprojecting cells contacted by STT boutons among all LYfilled SI-projecting cells in the present study is much lower than the percentage (about 24%) of the cells overlapping STT terminals of all retrogradely labeled SI-projecting cells in our earlier studies. The difference between the present study and the earlier studies must be due to the completely different methods. In Gingold et al. (19911, only the somata or nuclei of the SI-projecting cells were retrogradely labeled and the STT afferents were anterogradely labeled with WGA-HRP. Unlike the BD technique which clearly labels the profiles of terminal structures, in WGA-HRP material it is difficult to distinguish the terminal boutons from the axons or fibers. Furthermore, in the Gingold et al. study (1991) the SI-projecting cells were counted as STT overlapping cells when the labeled STT afferents were located within 100 pm from the neuronal somata. In the present study, synaptic contacts are identified as only those structures where a bouton was attached to a labeled neuronal dendrite without a visible gap between them (examined T. SHI AND A.V. AF'KARIAN under lOOx objective). Whether or not the contacts are actual synapses can only be proven with electron microscopy. We have tried to further identify these contacts at the electron microscopic level, but the issue preservation was not adequate. Moreover, we could not see the BD-labeled terminals in the same section during LY injections. Therefore, it was difficult to always fill the cells closest to the BD-labeled terminals. We conclude that this is one of the reasons why only a small number of cells were found contacted by STT terminals. The results show that the neurons in VPL, VPI, and PO had few STT terminal-dendritic contacts, implying that the STT input is highly divergent in these nuclei. The SIprojecting neurons in CL seemed to have more STT contacts than the VPL or VPI neurons, which suggests that CL is an important nucleus in relaying STT inputs to SI. CONCLUSIONS In the present study, both the SI-projecting neurons in VPL, VPI, Pulo, PO, CL, CM, and VL and the STT terminals were well labeled. SI-projecting neurons in VPL, VPI, CL, and PO were found to have contacts with STT terminal boutons, indicating that the method used to stain the entire identified thalamocortical neuron and the anterogradely labeled terminals is useful for studying the morphology of multisynaptic structures. The quantitative analyses and comparisons of the neuronal somata and dendrites between the various thalamic nuclei revealed that the SI-projecting neurons in these different nuclei were rather similar in the soma and dendritic morphology with some exceptions: 1)VL neurons had the largest soma sizes, the most spherical soma shapes, the most numbers of the primary dendrites, and the greatest total dendritic length among all of the studied neurons. 2) Pulo neurons also had more primary dendrites and longer total dendritic lengths than the neurons in VPL, VPI, CL, PO, and CM. 3) The SI-projecting VPL and VPI neurons were different in their soma sizes, shape factors, and orientations. The superficial SI-projecting cells in VPL were smaller than the rest of SI-projecting cells in VPL. However, the VPI cells projecting to superficial SI were not different from the VPI cells projecting to all SI layers. These results indicate that although there were some differences in the morphology of SI-projecting cells in the different thalamic nuclei, morphological similarities existed among SI-projecting neurons. Actually, VPL, VPI, and CL neurons projecting to SI were similar in dendritic morphology and branching pattern, and varied from PO, CM, and VL neurons projecting to SI. The first group of nuclei share common inputs (medial lemniscal and STT) and some common cortical targets (SI and SII). The inputs to the second group are from more diverse regions (e.g., inferior colliculus, STT, dentate nucleus, globus pallidus), and their outputs are also more divergent (e.g., SI, insular, auditory, and motor cortices). Therefore, it is implied that both the afferent inputs and efferent targets may, at least partially, affect the determination of detailed dendritic branching pattern of cortically projecting neurons. It should be emphasized, however, that this hypothesis remains to be rigorously tested by examining morphologic differences between cells with different input and output connectivities, within a given nucleus (or region). MORPHOLOGY OF SI-PROJECTING THALAMIC CELLS ACKNOWLEDGMENTS We thank Dr. C. Brandon for providing antibodies, Dr. D.R. Chialvo for help regarding fractal analysis, and Dr. J.A. Robson for providing a computerized microscope. The technical help of R.T. Stevens, H.M. Newman, and B.R. Krauss is appreciated. This research was funded by the Department of Neurosurgery at SUNY HSC. LITERATURE CITED Apkarian, A.V., and T. Shi (1994) Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J. Neurosci. 14:6779-6795. Bentivoglio, M., M. Molinari, D. Minciacchi, and G. Macchi (1983) Organization of the cortical projections of the posterior complex and intralaminar nuclei of the thalamus as studied by means of retrograde tracers. In M. Macchi, A. Rustioni, and R. Spreafico (eds): Somatosensory Integration in the Thalamus. Amsterdam: Elsevier Science Publishers, pp. 337-363. Brandon, C. (1985) Improved immunocytochemical staining through the use of Fab fragments of primary antibody, Fab-specific second antibody, and Fab-horseradish peroxidase. J. Histochem. Cytochem. 33:715-719. Brandon, C., and M.H. Criswell (1991) Antiserum to Lucifer Yellow: preparation, characterization, and use for immunocytochemical localization of dye-filled retinal neurons. J. Histochem. Cytochem. 39: 15471553. Brandt, H.M., and A.V. Apkarian (1992) Biotin-dextran: a sensitive anterograde tracer for neuroanatomic studies in rat and monkey. J. Neurosci. Methods 4 5 3 5 4 0 . Buhl, E.H. (1993) Intracellular injection in fixed slice in combination with neuroanatomical tracing techniques and electron microscopy to determine multisynaptic pathways in the brain. Microsc. Res. Tech. 24t15-30. Buhl, E.H., and J. Lubke (1988) Intracellular Lucifer yellow injection in fixed brain slices combined with retrograde tracing, light and electron microscopy. Neuroscience 28:3-16. Buhl, E.H., W.K. Schwerdtfeger, P. Germroth, and W. Singer (1989) Combining retrograde tracing, intracellular injection, anterograde degeneration and electron microscopy to reveal synaptic links. J. Neurosci. Methods 29:241-250. Cajal, S.R. (1911) Histologic du Systeme Nerveux de L’homme et des VertebrBs. Paris: Maloine. Chiaia, N.L., R.W. Rhoades, S.P. Fish, and H.P. Killackey (1991) Thalamic processing of vihrissal information in the rat: 11. Morphological ventral posterior nucleus and posterior nucleus neurons. J. Comp. Neurol. 314:217-236. Cusick, C.G., and H.J.I. Gould (1990) Connections between area 3b of the somatosensory cortex and subdivisions of the ventroposterior nuclear complex and the anterior pulvinar nucleus in squirrel monkeys. J. Comp. Neurol. 29283-102. Dong, W.K., H. Ruy, and I.H. Wagman (1978) Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J. Neurophysiol. 41: 1592-1613. Dostrovsky, J.O., and G. Guilbaud (1990) Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain 40:93-104. Fenelon, G., J. Yelnik, C. FranGois, and G. Percheron (1994) Central complex of the primate thalamus: a quantitative analysis of neuronal morphology. J. Comp. Neurol. 343:463479. Friedman D.P., and E.G. Jones (1981) Thalamic input to areas 3a and 2 in monkeys. J. Neurophysiol. 45:59-85. Friedman, D.P., and E.A. Murray (1986) Thalamic connectivityof the second somatosensory area and neighboring somtosensory fields of the lateral sulcus of the macaque. J. Comp. Neurol. 252348-373. Gingold, S.I.,J.D. Greenspan, and A.V. Apkarian (1991) Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between sphinothalamic terminals and thalamocortical cells in squirrel monkeys. J. Comp. Neurol. 308:467490. Guillery, R.W. (1966) A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J. Comp. Neurol. 128:21-50. Harris, R.M. (1986) Morphology of physiologically identified thalamocortical relay neurons in the rat ventrobasal thalamus. J. Comp. Neurol. 251t491-505. 23 Havton, LA., and P.T. Ohara (1993) Quantitative analyses of intracellularly characterized and labeled thalamocortical projection neurons in the ventrobasal complex of primates. J. Comp. Neurol. 336.135-150. Havton, L.A., and P.T. Ohara (1994) Dendritic orientation of thalamocortical projection neurons in the ventrobasal complex of macaques. Brain Res. 638:126-132. Hazlett, J.C., C.R. Dutta, and C.A. Fox (1976) The neurons in the centromedian-parafascicular complex of the monkey (Macaca mulatta): a Golgi study. J. Comp. Neurol. 168:41-73. Jones, E.G. (1985) The Thalamus. New York: Plenum Press. Jones, E.G., and D.P. Friedman (1982) Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J. Neurophysiol. 48t521-544. Jones, E.G., and R.Y. Leavitt (1974) Retrograde axonal transport and the demonstration of nonspecific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J. Comp. Neurol. 154:349-77. Jones, E.G., S.P. Wise, and J.D. Coulter (1979) Differential thalamic relationships of sensory-motor and parietal cortical fields in monkeys. J. Comp. Neurol. 183:833-882. Kaas, J.H., and T.P. Pons (1988) The somatosensory system in primates. Comp. Prim. Biol. 4:421468. Krauss, B.R., B.J., Serog, D.R. Chialvo, and A.V. Apkarian (1994) Dendritic complexity and the evolution of cerebellar purkinje cells. Fractals 295-102. Kultas-Ilinsky, K., and LA. Ilinsky (1991) Fine structure of the ventral lateral nucleus (VL) of the Macaque mulatta thalamus: cell types and synaptology. J. Comp. Neurol. 344:319-349. Kumazawa, T., K. Mizumura, J. Sato, and M. Minagawa (1989) Facilitatory effects of opiods on the discharges of visceral nociceptors. Brain Res. 497.231-238. Maranto, A.R. (1982) Neuronal mapping: a photooxidation reaction makes Lucifer Yellow useful for electron microscope. Science 21 7t953-955. Nomura, T., N. Nishikawa, and T. Yokota (1992) Intracellular HRP study of nociceptive neurons within the ventrobasal complex of the cat thalamus. Brain Res. 570:323-332. Ohara, P.T., and L.A. Havton (1994) Dendritic architecture of rat somatosensory thalamocortical projection neurons. J. Comp. Neurol. 341:159-171. Penny, G.R., D. Fitzpatrick, D.E. Schmechel, and I.T. Diamond (1983) Glutamic acid decarboxylase-immunoreactive neurons and horseradish peroxidase-labeled projection neurons in the ventral posterior nucleus of the cat and Galago senegalensis. J. Neurosci. 3:1868-1887. Peschanski, M., C.L. Lee, and H.J.I. Ralston (1984) The structural organization of the ventrobasal complex of the rat as revealed by the analysis of physiologically characterized neurons injected intracellularly with horseradish peroxidase. Brain Res. 197:63-74. Pons, T.P., and J.H. Kaas (1985) Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. J. Comp. Neurol. 240:16-36. Ralston, D.D., and H.J.I. Ralston (1992) Medial lemniscal and spinalprojections to the macaque thalamus: a n electron microscopic study of differing GABAergic circuitry serving thalamic somatosensory mechanisms. J. Neurosci. 14:2485-2502. Ralston, H.J., M. Peschanski, and D.D. Ralston (1985) Fine structure of spinothalamic tract axons and terminals in rat, cat, and monkey demonstrated by the orthograde transport of lectin conjugated to horseradish peroxidase. In H.L. Fields, R. Duhner, and F. Cervero (eds): Advances in Pain Research and Therapy. New York: Raven Press, pp. 269-275. Rausell, E., and C. Avendano (1985) Thalamocortical neurons projecting to superficial and to deep layers in parietal, frontal and prefrontal regions in the cat. Brain Res. 347:159-165. Rausell, E., C.S. Bae, A. Vinnuela, G.W. Huntley, and E.G. Jones (1992) Calbindin and parvalhumin cells in monkey VPL thalamic nucleus: distibution, laminar cortical projections, and relation to spinothalamic terminations. J. Neurosci. 12:40884111. Shi T., R.T. Stevens, J. Tessier, and A.V. Apkarian (1993) Spinothalamocor. tical inputs nonpreferentially innervate the superficial deep cortical layers of SI. Neurosci. Lett. 160.209-213. Stevens, R.T., S.M. London, and A.V. Apkarian (1993) Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res. 631 ~241-246. Tombol, T. (1967) Short neurons and their synaptic relations in the specific thalamic nuclei. Brain Res. 3.307-326. 24 Tombol, T. (1969) Two types of short axon (Golgi 2nd) interneurons in the specific thalamic nuclei. Acta. Morphol. Hung. 175'85-297. Tombol, T., M. Bentivoglio, and G. Macchi (1990) Neuronal cell types in the thalamic intralaminar central lateral nucleus of the cat. Exp. Brain Res. 81:491-499. Winer, J.A., and D.K. Morest (1983) The neuronal architecture of the dorsal division of the medial geniculate body of the cat: A study with the rapid Golgi method. J. Comp. Neurol. 221:l-30. Wouterlood, F.G., B. Jorritsma-Byham, and P.H. Goede (1990) Combination of anterograde tracing with Phaseolus uulgarzs-leucoagglutinin, retrograde fluorescent tracing and fixed-slice intracellular injection of Lucifer yellow. J. Neurosci. Methods 33.207-217. Yamamoto, T., T. Noda, M. Mitata, and Y. Nishimura (1984) Electrophysiological and morphological studies on thalamic neurons receiving entope- T. SHI AND A.V. APKARIAN dunculo- and cerebello-thalamic projections in the cat. Brain Res. 301r231-242. Yamamoto, T., T. Noda, A. Samejima, and H. Oka (1985a) A morphological investigation of thalamic neurons by intracellular HRP staining in cats. J. Comp. Neurol. 236:331-347. Yamamoto, T., A. Samejima, and H. Oka (1985b)An intracellular analysis of the entopeduncular inputs on the centrum medianum-parafascicular nuclear complex in cats. Brain Res. 348:343-347. Yen, C., and E.G. Jones (1983) Intracellular staining of physiologically identified neurons and axons in the somatosensory thalamus of the cat. Brain Res. 280:148-154. Yen, C., M. Conley, and E. G. Jones (1985) Morphological and functional types of neurons in cat ventral posterior thalamic nucleus. J. Neurosci. 5:1316-1338.