* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1. Twelve equal charges q are situated in the corners of a regular 12
Survey
Document related concepts
Transcript
This section contains 30 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which
ONLY ONE is correct.
1. Twelve equal charges q are situated in the corners of a regular 12 sided polygon and a
test charge Q is placed at the centre of the polygon. Now one of the 12 q’s replaced by
an equal and opposite charge –q .Then what will be the force on Q assuming r is the
distance from centre to each charge q?
A)
B)
C)
D)
2. The electric field at a distance Z above the centre of a circular loop of radius R which
carries a linear charge density λ is KZ / (R2+Z2)1.5. Then K is
A) λR /6ϵ0
B) λR /4ϵ0
C) λR /3ϵ0
D) λR /2ϵ0
3. A body moves from r1= 2i – 3j – 4k meters to r2 = 3i – 4j + 5k meters under the influence
of an external force F = 4i + j + 6k Newton. The absolute value of work alone done by
this force is
A) 52.5 J
B) 57 J
C) 53 J
D) 48 J
4. Two tall buildings are 30m apart. The speed with which a ball must be thrown
horizontally from a window 150m above the ground on one building so that it enters a
window 27.5m from the ground in the other building is
A) 6 m/s
B) 4.5 m/s
C) 7m/s
D) 4m/s
5. Two small spheres of equal masses start moving in opposite directions from a point A in
a horizontal Circular orbit with tangential velocities v and 2v respectively. Between
collisions the spheres move with constant speeds. The number of elastic collisions the
spheres will make before they reach the point A again is
A) 2
B) 3
C) 4
D) 6
6. If angular momentum of an electron revolving in a circular orbit is L then it’s magnetic
moment is (where m is the mass of the electron and e is the charge)
A) eLm
B) eL/m
C) eL/2m
Page 1 of 15
D) eL2/m
7. Three energy levels A, B, C of an atomic system are such that EA < EB < EC . If the
wavelengths corresponding to transmission C→B, B→A and C→A are λ1, λ2 and λ3
respectively, then
A) λ1 +λ2 + λ3 =0
B) λ1 +λ2 = λ3
C) λ3 =( λ1λ2 )/( λ1 +λ2 )
D) λ1 2+ λ22 = λ32
8. A Spherical volume of radius R with its center at the origin contains a uniform charge
density ρ. A spherical cavity of radius a (a < R) centered at a point C, is scooped out of
the sphere such that that there is no charge in the cavity. What is the magnitude of
electric field within the cavity at a distance b (b<A) from the center of the cavity?
A) ρ ⃗ /3ϵ0
B) ρ /3ϵ0
C) ρ(
⃗ )/3ϵ0
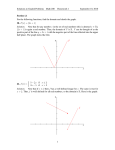
D) ρ(
⃗ )/3ϵ0
9. What is the capacitance per unit length of two coaxial metallic cylindrical tubes of radii
a & b where b>a?
A) 1.5 πϵ0 / ln(b/A)
B) 2πϵ0 / ln(b/A)
C) 4πϵ0 / ln(b/A)
D) 6πϵ0 / ln(b/A)
10. A thin isolated and insulated wire forms a planner spiral that closely packed and has a
large number of turns (N number of turns). The inner and outer radii of the spiral are a
& b respectively. A steady current I flow in the wire. The magnetic field at the centre of
the spiral is K ln(b/A) . Then value of K is?
A) μ0IN/2(b-A)
B) μ0IN/2(b+A)
C) 3μ0IN/2(b-A)
D) 3μ0IN/2(b+A)
11. If potential between A and B is V, find the current in AC. Each resistor has resistance R.
A) V/5R
B) V/4R
C) V/2R
D) V/R
Page 2 of 15
12.The Voltage over a cycle varies as
v = V0
for 0 ≤ t ≤ π/ɷ
v= - V0
for π/ɷ ≤ t ≤ 2π/ɷ
The average value of voltage over the cycle is
A) V0/√
B) V0/2
C) Zero
D) 2V0/π
13.Find the maximum angle that can be made in glass medium (μ = 1.5) if a light ray is
refracted from glass to vacuum?
A)
B)
C)
D)
14.A particle is moving towards the fixed spherical mirror. The image must
A) Must move away from the mirror
B) Must move toward the mirror
C) May move toward the mirror
D) Will move toward the mirror, only if mirror is convex.
15.Visible photons are characterized by the wavelength of the range of 400 nm -800 nm.
The corresponding energy range is about
A) 1.55 eV to 3.09 eV
B) 15.5 eV to 30.9 eV
C) 9.08 eV to 15.03 eV
D) 0.1 eV to 1 eV
16.The de-Broglie wavelength of an electron in the ground state of an atom is λ, then what
is the circumference of nth orbit of hydrogen atom
A) 2πnλ
B) 2πn2λ
C) nλ
D) n2λ
17. A solid cube has mass of 2.23 kg and side measuring 1.1 m each. Value of density is
A) 1.68 kg/m3
B) 1.71 kg/m3
C) 1.6 kg/m3
D) 1.7 kg/m3
18. Two blocks with masses m1=1 kg and m2=2 kg are connected by a spring of spring
constant 24 N/m and placed on a frictionless horizontal surface. The block m1 is
imparted an initial velocity 12 cm/s to the right. The amplitude of oscillation is:
A) 2 cm
B) 1 cm
C) 3 cm
Page 3 of 15
D) 4 cm
19. A water tank of length 100 cm and height 40 cm contain water upto 30 cm. Now the
tank is accelerated in the forward direction as shown:
Maximum acceleration the tank can
gain so that the water inside doesn’t
spill out is (g=10 m/s2)
A) 1 m/s2
C) 2.5 m/s2
B) 2 m/s2
D) 4 m/s2
20. Six identical rods are connected as shown. The temperature of free ends A, B, C are
2000C, 200C and 200C respectively. The temperatures of junctions D, E and F are
respectively:
A) 1400C, 800C, 800C
B) 1550C, 1100C, 1100C
C) 1250C, 650C, 650C
D) 1250C, 750C, 750C
21. A U-tube manometer of uniform cross-section has one limb vertical and other inclined
at 300 to vertical. Both the limbs are open to atmosphere and contain mercury up to a
height of 30 cm. Water is poured in the vertical limb such that its column height is 20
cm. Calculate the length of mercury column in the inclined tube. (atmospheric pressure
= 76 cm of Hg, specific gravity of mercury = 13.6)
A) 34.64 cm
B) 35.43 cm
C) 36.75 cm
Page 4 of 15
D) 37.46 cm
22. 5 kg of steam is contained within a piston-cylinder assembly. The steam undergoes an
expansion process from state 1, where the specific internal energy is 2709.9 kJ/kg to
state 2, where the specific internal energy is 2659.6 kJ/kg. During this process 80 kJ of
heat is transferred to the steam and a paddle wheel transfers 18.5 kJ of work.
Determine the work done by the steam on the piston.
A) 61.5 kJ
B) 98.5 kJ
C) 350 kJ
D) 0 kJ
23. The Young’s Modulus of the material of a wire is 6 x 1012 N/m2 and there is no
transverse strain in it. Then its modulus of rigidity will be:
A) 1012 N/m2
B) 3 x 1012 N/m2
C) 2 x 1012 N/m2
D) 8 x1012 N/m2
24. The correct statements are:
A) A metallic ball has a spherical cavity at its center. If the ball is heated, the volume of
cavity will increase.
B) Two spheres are made up of the same material and have same radius, one is solid
and other is hollow. When heated to the same temperature, increase in diameter
will be same for both.
C) A body suspended from a spring balance is immersed in water. Then on heating the
liquid, the reading of spring balance reduces.
D) When a pendulum clock is heated, it runs faster.
25. A one litre flask contains some mercury. It is found that at different temperatures, the
volume of air inside the flask remains the same. The initial volume of mercury in the
flask is(α for glass=9 x 10-6/0C and γ for mercury=1.8 x 10-4 /0C)
A) 0.25 L
B) 0.3 L
C) 0.2 L
D) 0.15 L
26. A force F acts tangentially at the highest point of a disc of mass m kept on a rough
horizontal plane. If the disc rolls without slipping, the acceleration of centre of disc is
A) 2F/3m
B) 10F/7m
C) Zero
Page 5 of 15
D) 4F/3m
27. A satellite of mass m is orbiting around earth along a circular path of radius, r with a
uniform speed, v and period, T. Its total energy is E. Choose the wrong statement regard
to this satellite.
A) v α r -1/2
B) E α r -1
C) T2 α r3
D) T α m
28. The speed of sound in helium (He) at a certain temperature is 1420 ms -1. The speed of
sound in oxygen(O2) at the same temperature will be(assume both gases to be ideal)
A) 500 ms -1
B) 650 ms -1
C) 330 ms -1
D) 460 ms -1
29. When the height of the TV antenna in a TV station doubled the area of coverage of
transmission becomes
A) 2 times
B) 0.5 times
C) 4 times
D) √ π times
30. A 10 gm sample of a radioactive material decays to 9 gm in 6 days. After a further
elapse of 18 days the amount becomes
A) 34.5 %
B) 65.6 %
C) 51.5%
Page 6 of 15
D) 38.5%
This section contains 30 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which
ONLY ONE is correct.
31. An electrode is setup with Cr3+ and Cr2O72- each with 0.01M solution in acid medium
with Pt as electrode material at 25oC (Eo Cr2O72-/ Cr3+ = 1.33 V) then
(i) at pH = 1 E=1.21v
(ii) at pH = 3 E=0.94v
(iii) at pH = 1 E = 1.94v
(iv) at pH = 3 E=2.21v
Correct statement are
A) I & iv
B) I & II
C) II & III
D) All
32.
Me3C-CO-Cl
major product (A)
AlCl3
Then A is
A)
B)
C)
D)
33. Product A is,
1) HCN
2) LiAlH4
3) NaNO2
A)
B)
Major product (A)
C)
Page 7 of 15
D)
34. Which of the following is not planar?
A) Na3B3O6
B) I2Cl6
C) Sheet Silicate
D) Inorganic Graphite
35. An element has exceptional electronic configuration as 4d10 5s0. It belongs to
A) 4th period, 11th group
B) 5th period, 10th group
C) 4th period, 10th group
D) 5th period, 11th group
36. Which of the following ores contain copper as well as iron
A) Cuprite
B) Chalcocite
C) Chalcopyrite
D) Malachite
37. 50 ml of 0.1M aqueous acetic acid is titrated with 100ml of 0.1M aqueous NaOH
solution. pH of resulting solution is (pKa(CH3COOH) = 4.7)
A) 4.098
B) 4.302
C) 4.030
D) 5.030
38. In borax test for Cu2+, which non-metal changes blue colour of copper compound
(obtained when heated in flame) to colourless?
A) Nitrogen
B) Sulphur
C) Carbon
D) Chlorine
39. Which is not correct?
A) C2 molecule has 12 electrons out of which 8 electrons occupy bonding orbital while 4
electrons are in anti-bonding orbital.
B) C2 molecules have been found to exist in vapour phase
C) C2 molecule contains double bond and both are π bonds
D) C2 molecule is paramagnetic
40. Cr2O72- pH=X
CrO42- pH=Y Cr2O72X and Y can be
A) 4 & 8
B) 8 & 9
C) 8 & 4
D) 4 & 5
41. Positronium is a species consisting of an electron bound to a positron instead of
proton. If the radius of its 2nd orbital is Ro where Ro is radius of its Ist Bohr orbit of
hydrogen, then value of is
A) 4
B) 16
C) 8
D) 2
42. Two moles of an ideal gas (r=3/2) is made to expand reversibly in a path P
=8
where x=3/2. Volume became four times during the expansion. The change in entropy
of the system during the expansion is [R = 2 cal K-1 mol-1]
A) 5.6 cal K-1 mol-1
B) 11.2 cal K-1 mol-1
C) 2.8 cal K-1 mol-1
D) zero
43. When the following compound is heated in presence of KOH, the product will be,
Page 8 of 15
A)
B)
C)
D) None of these
44. Find the pH of 0.1 M CH3COOH solution having Vant Hoff factor of 1.01
A)1
B)2
C)3
D)4
45. For the reaction
( )
( )
degree of dissociation is 0.2 at 1atm pressure Kp is
A) 6
B) 36
C) 1/6
D) 1/36
46. For a particular reaction with initial concentration of reactant as a1 & a2, the half-life
period are t1 and t2 respectively. Order of reaction (n) is given by
A)
C)
( )
( )
( )
B)
( )
( )
( )
D) None of these
47. Which of the following is a correct order of crystal field splitting of ligands?
A) OH- > F- > NO3B) S2- < Br- < IC) CO < CN- < NO2D) NO3- > F-> OH48. Chloroform is slowly oxidised by air in the presence of light to form
A) formyl chloride
B) formic acid
C) phosgene
D) trichloroacetic acid
49. Which of the following monomers will give free radical polymerization most rapidly:
A) CH2= CH2
B) C6H5CH=CH2
C) CH3-CH=CH2
D) CH3-C(CH3)=CH2
Page 9 of 15
50. The pKa of carboxylic group in Valine is 2.31 and pKa for amino group is 9.69. Isoelectric
point of Valine is
A) 12
B) 6
C) 3
D) 4
51. In the reaction
(aq)
(aq) +
(aq), the equivalent mass of
ion is
(m=molecular mass of ClO )
A) M/4
B) M/2
C) M/6
D) M/3
52. Increasing order of basic strength of aniline and substitute anilines is
I
II
III
A) III>II>IV>I
B)II>III>IV>I
C) I>IV>II>III
D) I>II>III>IV
IV
53. When K2Cr2O7 is heated with conc. H2SO4 and NaCl, the orange vapour evolved is
A) Cr2O3
B) CrCl3
C) Na2Cr2O7
D) CrO2Cl2
54. The pair of salts which can react with KOH is
A) K2HPO4 & KH2PO3
B) K2CO3 & K2SO4
C) KHPO3
D) K2HPO4 & KH2PO2
& KH2PO2
55. How many geometrical isomers are possible for the given compound?
A) 8
B) 4
C) 2
D) No geometrical isomerism
56. In the test of Sulphur, Violet colour of sodium thionitroprusside is formed, what is the
formula of sodium thionitroprusside
A) Na[(CN)5NO]
B) Na2[Fe(CN)5NOS]
C) Na4[Fe(CN)5NOS]
D) Na4[Fe(CN)6NO]
Page 10 of 15
57. Density of palladium (At. Wt 106.4) is 12.0g/cc. Unit cell length is 3.9x10-8cm. The
effective no of atoms in unit cell is (NA=6x1023)
A) 2
B) 3
C) 4
D) 1
58. Which of the following antibiotics has the broadest spectrum of action?
A) Penicillin
B) Erythromycin
C) Chloramphenicol
D) Tetracycline
59. The ancient Egyptians knew that feeding a person liver would cure him/her of night
blindness. What active component of liver is effective in curing night blindness?
A) Retinol
B) Folic acid
C) Thiamine
D) Ascorbic acid
60. Which two of the following compounds represents a pair of enantiomers?
A) I & II
B) II & III
C) III & IV
Page 11 of 15
D) II & IV
This section contains 30 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which
ONLY ONE is correct.
61. Considering a function y = x-[x] where [x] represents greatest integer function, which of
the following statements is true?
A) y(x) is continuous everywhere
C) y(x) is discontinuous everywhere
B) y(x) is continuous at integers
D) y(x) is discontinuous at integers
62. Let S1 , S2 , . . . be such that for each n≥1 the length of a side of Sn equals the length of a
diagonal of Sn+1 . If the length of a side S1 is 10cm, then for which of the following value
of n is the area of Sn less than 1 sq.cm?
A) 5
B) 6
C) 7
D) 8
63. If a variable takes value 0 , 1 , 2 , . . . . . . , n with frequencies 1, nC1, nC2, . . . . . , nCn,
then the A.M is?
( )⁄
A) n
B)
C)
D) None of these
64. Cone is maintained at a radius 10 cm and is stretched from top to increase the height. If
the semi angle of cone is 450 and the angle is decreasing at 1.5 0/Sec. The rate of
increase of height of the cone is?
A) 20cm/s
B) 30cm/s
C) 20√ cm/s
65. Let a, b, c be pth , qth ,rth terms of a GP.
Find the angle between vectors, ⃗
(
(
)̂
(
A)
)̂
B)
(
)̂
(
)̂
D) 30√ cm/s
(
) ̂ and
)̂
C)
66. The dual of the statement [p v (~q)] ^ (~p) is
A) p v (~q) v (~p) B) (p ^ ~q) v (~p) C) p ^ ~q v ~p
D) π
D) none of these
67. Equation of the smallest circle passing through the intersection of line x + y=1 and the
circle x2+y2 = 9 is:
A) x2+y2+x+y-8=0
C) x2+y2-x+y-8 =0
B) x2+y2-x-y-8=0
D) none of these
Page 12 of 15
68. Degree of the given differential equation is:
x= ∑
(
)
A) n
B) 1
C) Not defined
D) None of these
69. Evaluate: ∑
A) π + tan -1(-3)
C) π + tan -1( )
B) tan -1( )
D) tan -1(
)
70.
( )
{
( )
{
Function f + g is :
A) one-one and on-to
C) Many one and on-to
B) one-one - but not on-to
D) Neither one-one nor on-to
71. The value of ∑
A)
(
B) -1
72. If sin (α+β) = 1 , sin(α-β) =
A) 1
) ,where = √
C) -
equals
D) 0
then tan(α+2β)tan(2α+β) is equal to
B) -1
C) 0
D) 2
73. If B is a non-singular matrix and A is a square matrix, then Det (B-1AB) is equal to
A) Det (B)
B) Det (A)
C) Det (B-1)
D) Det (A-1)
74. Area enclosed between curve y2=2x and its mirror image along the y-axis within
x2 + y2 = 8 is:
A) 2π -
B) π -
C) 4π -
D) 3π – 2
75. Find a point on the curve x2+2y2=6, whose distance from x + y= 7 is minimum:
A) (2, 1)
B) (√ ,√ )
C) (√ ,√
)
D) (1,√ )
76. If a matrix A is such that 3A3 + 2A2 + 5A + I = 0 , then A-1 is equal to:
A) 3A2 + 2A + 5I
C) 3A2 - 2A + 5I
B) –(3A2 + 2A + 5I)
D) None on these
Page 13 of 15
77. Ten different letters of an alphabet are given, words with five letters are formed from
these given letters then the number of words which have at least one letter repeated is
A) 69760
B) 30240
C) 99784
D) None of these
78. If x + y + z = xyz , then tan-1x + tan-1y + tan-1z =
A) π
79. Find
B)
, where y =√
A)
C) 1
√
B)
D)
√
C)
√
[
]
√ (√
√
(
[
D)
)
]
80. Let A and B be any 2 fixed points and P another point in the plane, moves in such a way
that k1PA + k2PB =k3, where k1, k2 and k3, are real constants. Regarding Locus of P which,
of the following is NOT true
A) a circle if k1 = 0, & k2, k3 > 0
B) a circle if, k1 > 0, k2 < 0 & k3 = 0
C) an ellipse if k1 = k2 > 0 & k3 > 0
D) a hyperbola if k2 = -1 and k1, k3 >0
81. The plane x + 2y – z = 4 cuts the sphere x2 + y2 + z2 – x + z – 2 = 0 in a circle of radius
A) √
82. Integrate: ∫
A)
C)
B) 2
√(
C) 1
D) 3
)
B)
√
D)
√
√
√
)
83. Length of latus rectum of the parabola: 2((
A) 2a
84. Let the line
A) (6,-17)
C) √
B) 4a
(
) )=(
)
D) 2√
lie in the plane x + 3y – αz + β = 0. Then (α,β) equals
B) (-6,7)
C) (5,15)
D) (-5,5)
85. A line AB is given by x + y =1, where A and B are points in 4th quadrant. AB is reflected
by a mirror located along 2x+y =1. Equation of the image of AB is,
A) 6x+y=1
B)7x+y=1
C) 8x+y=1
D) 9x+y=1
Page 14 of 15
86. Solve the differential equation (4x + 2y +5) = (2x+y-1)
A) 7 ln |10x+5y+9| + 10y – 5x = C
B) 5 ln |7x+10y+9| + 7y – 10x = C
C) 7 ln |7x+10y+9| + 7y – 10x = C
D) 5 ln |10x+5y+9| + 10y – 5x = C
87. If (1+2x+3x2)10 = a0 + a1x + a2x2 + . . . . . . . . + a20x20
Then a1 is equal to?
A)10
B)20
C)210
D)None of these
88. If p and q are the roots of the equation x2 + px + q = 0 , then
A) p = 1
B) p = 1 or 0
C) p = -2
D) p = -2 or 0
89. The number of values of x in the interval [0,3π] satisfying the equation
2sin2x + 5sinx – 3 = 0 is
A) 6
B) 1
C) 2
D) 4
90. The numbers 1 , 2 , 3 , . . . . . . , n are arranged in a random order. The probability that
the digits 1 , 2 , 3 , . . . . . . , k (k<n) appear as neighbours in that order is
A)
B)
C)
(
)
Page 15 of 15
D) None of these