* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy Levels in Atoms
Survey
Document related concepts
Transcript
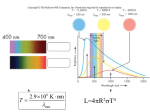
The Small Angle Approximation The 2p shows the fact that the natural units for angle here are “radians”; 2p of them for a full circle (so 57.3 degrees per radian). Measuring distance with triangulation Parallax – direct distances to the stars d(parsec) = 1/p(arcsec) ; 1 parsec = 3.26 light years The Luminosity Law The intrinsic luminosity of a star depends on its temperature (because blackbody radiation goes like T4 per unit area, and its size (to account for its total area ~R2) : LR T 2 l1 4 l2 L1 R2 R1 T1 L2 T2 Observation of stellar properties You can tell something from the brightness and color of a star, but there are complications. •The color gives you an approximate temperature. •It is hard to tell the distance from the brightness, because the size is also quite variable: •A small blue star can be as luminous as a larger red star. A blue star can be the same brightness as a red star if they have the same size but the red star is much closer. •If you get the distance from parallax, then you can tell the size from the color and apparent brightness. •Since most stars are many light years away, their parallaxes are small fractions of an arcsec. •Observing from space helps with this. •Their sizes are generally too small to be measured directly (much smaller fractions of an arcsec). •Interferometry will help with this. The Inverse-Square Law The apparent brightness of a source goes down like the square of the distance to the source (and depends on the intrinsic luminosity of the source : b L 2 d Energy Levels in Atoms We can think of an atom as consisting of a positive nucleus (protons and neutrons) surrounded by negative electrons. The electrons can be thought of as “orbiting” the nucleus, but are only allowed in certain orbits (or energy levels). A photon with exactly the right energy can excite the electron from one level to another. The electron will drop back to the “ground state”, and emit photons with specific energies as it does so. Energy transitions and photons The energy of photon that can interact with a level jump just depends on the energy difference between the levels. Levels can be skipped. Unique Atomic Signatures Each atom has a specific set of energy levels, and thus a unique set of photon wavelengths with which it can interact. Astro Quiz The spectra from two different atoms are observed. A spectral line in the first atom has exactly one-half the wavelength of a spectral line in the second atom. Which of the below is TRUE? • The spacing between 2 levels in the first atom must be twice the spacing between 2 levels in the second atom. • The spacing between 2 levels in the first atom must be half the spacing between 2 levels in the second atom. • The energy of the upper of 2 levels in the first atom must be half that of the energy of the upper of 2 levels in the second atom. Spectrometers A spectrometer makes an image (usually of its entrance slit) at every wavelength. A telescope illuminates the slit with starlight. Diffraction gratings Very fine facets or grooves will act like many little slits and produce an interference pattern that spreads wavelengths. far infrared grating Transitions in Hydrogen Hydrogen is particularly important and distinctive. The “Halpha” line is the one from levels 2-3; the Balmer series from level 2 is in the visible. The Lyman series lines are all ultraviolet. The Solar Spectrum Stellar Spectral Types Different stars show different spectral lines, or different line strengths. They were sorted by this appearance (Annie Jump Cannon). Decoding Stellar Spectra The reasons why spectra change were a bit subtle, so the ordering of the letters got scrambled when the temperature sequence was determined. Properties of the Spectral Types Most stars will be in their stable hydrogen-burning phase, called the “main sequence”. For these, the spectral type has a simple relation to the star’s properties. Note that luminosity has a large variation. The Brightest and Nearest Stars Towards the “HR diagram” Basketball player Height Adults Skinny Anorexic Fat Sumo wrestler Children Weight The Hertzsprung-Russell Diagram Hertzsprung and Russell had the idea of plotting the luminosity of a star against its spectral type. This works best for a cluster, where you know the stars are all at the same distance. Then apparent brightness vs spectral type is basically the same as luminosity vs temperature. They found that stars only appear in certain parts of the diagram. Basics of the HR diagram Size Mass Red dwarfs Size/Luminosity Hot stars are very bright but very rare. They can affect the light, but not the mass of the Galaxy. Red supergiants are more common.