* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neurophysiology of Pain - International Pain School
Proprioception wikipedia , lookup
Time perception wikipedia , lookup
Electrophysiology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Single-unit recording wikipedia , lookup
Metastability in the brain wikipedia , lookup
End-plate potential wikipedia , lookup
Synaptogenesis wikipedia , lookup
Development of the nervous system wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Signal transduction wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neural engineering wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Microneurography wikipedia , lookup
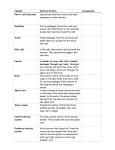
International Pain School Neurophysiology of Pain type in your name type in name of your institution The Scream Edvard Munch 1893 Lecture Objectives • Basics of the nervous system • Synaptic function • Nerve impulses • Transduction of peripheral painful stimuli • Central pathways • Control or modulation of pain signals • Pathophysiology of pain signaling pathway Definition of pain • "Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage". (The International Association for the Study of Pain) Epidemiology of pain • Pain is a global health issue (World Health Organization). • There is lack of accurate statistics but ~ tens of millions of people worldwide experience pain every year. Epidemiology of pain (cont.) • Post-surgical pain –About 240 million major surgeries take place annually worldwide. –~ 50% of patients report moderate to severe pain. • Chronic pain –1.5 billion people worldwide are diagnosed with chronic pain; • ~ 20% of Europeans suffer from chronic pain (Breivik et al. 2006). –3 - 4.5% of the global population suffers from neuropathic pain. • The incidence rate increases with age. Epidemiology of pain (cont.) • Cancer- & HIV/AIDs - related pain • 10 million people worldwide who are diagnosed with some form of cancer each year. • 1/3 of adults treated for cancer and 2/3 with advanced disease experience pain. • "Unrelieved cancer pain is a cause of major worldwide suffering, not because we don't have the tools necessary to relive pain, but because most patients don't have access to the essential pain-relieving medication“. (N. Cherney, European Society for Medical Oncology, 2012). The Nervous System • It is important to know the basic structure of the nervous system. • This will help in – understanding the mechanism by which nociceptive signals are produced. – know the different regions of the nervous system involved in processing these signals. – learn how the different medications and treatment for pain management work. Nervous System Central nervous system (CNS) •Brain and Spinal Cord Peripheral Nervous System (PNS) •Nerve fibers go to all parts of the body. •Send signals to the different tissues and send signals back to the CNS. Nerve Cells • The nervous system is made up of nerve cells which send long processes (axons) to make contact with other cells. Dendrites which receive signals from other nerve cells Cell Body Nerve ending that connect to other cells. Also act of receptors for stimuli Node of Ranvier Schwann Cell Myelin Sheath Cell nucleus Nerve Cell-to-Nerve Cell Communication Nerve cells communicate with other cells by releasing a chemical from the nerve endings – Neurotransmitters The Synapse. Basic Steps in Synaptic Transmission Synaptic Transmission Steps in the passage of signal from one nerve cell to other. Drugs are used to block the transmission of signals from one nerve cell to other. These drugs can effect 1.Ca2+ ion channel to prevent Ca2+ inflow which is essential for neurotransmitter (NT) release, e.g., the action of gabapentin. 2. Release of NT. 3. Prevent NT from binding to its receptor so stop further transmission of the signal. Electrical impulse • Signals move along a nerve process (axon) as a wave of membrane depolarization called the Action Potential. • The inside of all nerve cells has a negative electrical potential of around – 60 mV. • When stimulated this negative electrical potential becomes positive and then negative again in milliseconds. • The action potential moves along the nerve process (axon) to the nerve ending where it cause release of NT. Action Potential • When there is no stimulation the membrane potential is at its Resting Potential. • When stimulated, channels in the nerve membrane open allowing the flow of sodium ions (Na+) or calcium ions (Ca2+) into the nerve or cell. This makes the inside less negative and in fact positive -the peak of the action potential (+40 mV). • These channels than close and by the opening of K+ channels the membrane potential returns to its resting level. Stopping Action Potentials to Stop Nociceptive stimuli • Nociceptive stimuli are those that will create a sensation of pain after they are processed in the CNS. • Nociceptive signals can be prevented from reaching the CNS by blocking the action of the channels that control the movement of ions across the nerve membrane. • A number of anesthetic agents stop Na+ channel from working and hence stop the generation of actions potentials and transmission of signals to the CNS. • E.g. Procaine – local anesthetic agent. Sensory Systems • The sensory system that can be divided into two divisions: 1. A Sensory System that transmits innocuous stimuli such as touch, pressure, warmth. 2. A System that transmits stimuli that indicate that tissues have been damaged = nociceptive . • These two systems have different receptors and pathways in the PNS & CNS Skin Receptors Touch, pressure, vibration, skin stretch Neuroscience. 2nd edition. Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Sunderland (MA): Sinauer Associates; 2001. Nociceptors Nociceptors • Nociceptors are free nerve endings that respond to stimuli that can cause tissue damage or when tissue damage has taken place. • Present in membrane of free nerve endings are receptors (protein molecules) whose activity changes in the presence of painful stimuli. • (Note the use of the same term receptor is used for cell or organs or molecules that involved in transduction of a stimuli.) Transduction • Transduction is the process of converting the stimuli into a nerve impulse. • For this to occur the flow of ions across the nerve membrane has to change to allow entry of either Na+ or Ca2+ ions to cause depolarization of the membrane potential. • This involves a receptor molecule that either directly or indirectly opens the ion channels. Chemical agents … … which can cause the membrane potential at the free nerve ending (nociceptor) to produce an action potential. Agents Source Effect on nociceptor Potassium Damaged cells Activation Serotonin Platelets Activation Bardykinin Plasma kininogen Activation Histamine Mast cells Activation Prostaglandins Arachidonic acid from damaged cells Sensitization Leukotrienes Arachidonic acid from damaged cells Sensitization Substance P Primary afferents Sensitization from Fields HL. 1987. Pain. New York: McGraw-Hill. Summary of Transduction Process at the Periphery Chemical, Mechanical, Thermal stimuli Changes in the receptor Increase in ion flow across the membrane Depolarization of membrane potential (Generator potential) Action potential TRP Channels • Many stimuli – mechanical, chemical and thermal – give rise to painful sensation making transduction a complex process. • Recently receptor molecules have been identified Transient Receptor Potential (TRP) channels - that respond to a number of strong stimuli. • TRP receptors are also involved in transmitting the burning sensation of chili pepper. • In time, drugs that act on these receptors will be developed to control pain. Different TRP Channels Capsasin, the active ingredient in chili pepper, is used in patches for relief of pain. Menthol and peppermint gels are used to relieve muscle pain. Motor Output and Sensory Input to Spinal Cord Sensory nerves have their cell body outside the spinal cord in the dorsal root ganglia ( = 1st order neurons). One process goes to the periphery, the other goes to the spinal cord where it makes synaptic contact with nerve cells in the spinal cord ( = 2nd order neurons). The 2nd order neuron sends processes to other nerve cells in the spinal cord and to the brain. 2nd Order Nerve cells send nerve fibers in the spinal cord white matter Silverthorn Transmission of nociceptive signals from the periphery to the brain The spinal cord is more than a junction area for transmission of signals to the brain. There are spinal neural circuits, which can alter signal transmission. nociceptive stimulus to brain nociceptive stimulus Silverthorn A delta (d) and C nerve fibers • Nerve fibers are classified according to the: – (1) diameter of the nerve fiber and – (2) whether myelinated or not. • Ad and C nerve fiber endings respond to strong stimuli. • Ad are myelinated and C are not. • Action potentials are transmitted 10 times faster in the Ad (20 m/sec) fibers than in C fibers (2 m/sec). Ad and C fibers • Ad fibers respond mainly to mechanical and mechnothermal stimuli. • C fibers are polymodal, i.e. the nerve ending responds to several modalities – thermal, mechanical and chemical • This polymodal ability is due to the presence of different receptor molecules in a single nerve ending. Fast and Slow Pain C Ad • Most people when they are hit by an object or scrape their skin, feel a sharp first pain (epicritic) followed by a second dull, aching, longer lasting pain (protopathic). • The first fast pain is transmitted by the myelinated Ad fibers and the second pain by the unmyelinated C fibers. Central Pain Pathways • Nociceptive signals are sent to the spinal cord and then to different parts of the brain where sensation of pain is processed. • There are a pathways/regions for assessing the: 1. Location, intensity, and quality of the noxious stimuli 2. unpleasantness and autonomic activation (fightor-flight response, depression, anxiety). Intensity, Location, and Quality of Pain … … involve Spinothalamic and Trigeminal Pathways • The trigeminal pathway brings information from the face area. • The spinothalamic pathway brings information from the rest of the body. • Both these pathways project to the sensory cortex, which also receives information on innocuous stimuli such as touch, pressure and warmth via a separate pathway. Trigeminal pathway Spinothalamic pathway (Anterolateral Pathway) 2 Pain Transmission Pathways for location Intensity quality Neuroscience Purves et al. Unpleasant Quality and Autonomic Affective Motivational Pathway for Pain Spinal cord Parabrachial nucleus Amygdala (involved in fear) Hypothalamus (autonomic responses, e.g sweating) Thalamus Insula Anterior cingulate gyrus Frontal lobe Brain areas involved in processing of nociceptive signals Cerebellum Anterior cingulate gyrus Midbrain Frontal cortex The anterior cingulate and insula cortex are activated in human subjects … … in connection with an intense burning sensation following hand contact with the thermal grill. Adapted from Craig et al. 1994, 1996. From Principles of Neural Science, Kandell et al. Control of Pain Perception • There is difference between the objective and subjective aspects of injury and pain. • Despite similar injury, people can differ in how much pain they feel. • Depending on the context, pain may not be felt despite injury, e.g. battlefield injury, during intense sports. • This suggests that there is a physiological mechanism that controls the transmission of nociceptive signals to the brain or modifies the interpretation of pain. • The pain control system can also explain the placebo effect. Pain Modulation Pathway Nerve signals are sent form the somatic sensory cortex and hypothalamus to the periaqueductal gray matter (PAG). PAG sends signals to the parabrachial nucleus, medullary reticular formation, locus coeruleus, and Raphe neulei. These in turn can control the in the transmission of nociceptive signals from the spinal cord to the brain. This involves different involves different neurotransmitters. Endogenous Opioids • Internally produced molecules with opioid-like action which regulate transmission of nociceptive signals. • Three classes of these molecules have been identified. All are peptide molecules 1. Enkephalins 2. Endorphins 3. Dynorphins. • Despite these being powerful, endogenous modifiers of nociceptive signals, it has been difficult to produce and administer them in a way than can used in clinical practice. Location of nerve cells with endogenous opioid receptors • Spinal cord, Medulla, Periaqueductal gray matter (PAG) • In the spinal cord, endogenous opioids can prevent transmission between 1st order nerve cells (bringing signals from the periphery) and 2nd order spinal nerve cells that transmit the signals to the brain. • Also can prevent the increased synaptic efficiency, which plays a role in hyperalgesia. A photograph showing the presence in the spinal cord gray matter of endogenous opioids (bright area). (Center for Brain Research, Uni Vienna) Modulation of Pain Signal Transmission in the Spinal Cord Connections in the spinal cord where opiates act. Pain signals Spinal cord gray matters Block pain signals in the 2nd order neuron Neurotransmitters – serotonin (5-HT) and norephinephrine (noradrenaline) – in the spinal cord can block transmission of pain signals to the brain. Inflammatory Soup - Hyperalgesia • Tissue damage results in the release of a number of chemicals. • These increase nociceptors’ response to a stimulus (=hyperalgesia) & produce inflammation. • Hyperalgesia = when the magnitude of the response to a nociceptive stimulus is higher than normal. Julius-D & Basbaum-AI, Nature 2001;413:203 Clinical Application • Knowing the molecules involved in the “inflammatory soup” and how they are synthesized provides possible targets for pain reduction. • e.g. prostaglandins are produced by the COX enzyme. The activity of this enzyme is blocked by non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, diclofenac. Allodynia A mechanism for allodynia • A condition when normally non- Nociceptive signals from the periphery to spinal cord painful stimuli cause pain, e.g., touch, light pressure, cold. • Involves changes in the synaptic sensitivity of the nociceptive neurons in the spinal cord (central sensitization). NMDA (a receptor for glutamate) response increases in the spinal cord • Drugs such as ketamine, block NMDA receptors and so reduce transmisison of the nociceptive stimuli. Nociceptive nerve cells in the spinal cord now become responsive to non-painful stimuli Gate Control Theory of Pain • Mother says to child, “Come I will rub the area which is painful and this will make it feel better.” • After stubbing a toe, we instinctively rub the area; this reduces the sensation of pain. • Ronald Melzack and Patrick Wall in 1962 provided an possible explanation for this effect. Gate Theory Rubbing the area that hurts stimulates receptors of innocuous stimuli like touch, pressure and vibration. These mechano-receptors send signals along the Ab nerve fibers that (1) stimulate spinal nerves (inhibitory inter-neurons) that in turn inhibit signaling in the 2nd order neurons (projection neuron) and (2) directly inhibit the 2nd order neuron to reduce or stop pain signal from being sent to the brain (1) http://wikidoc.org/images/f/fe/Gate_control_A_firing.png (2) The Gate Control theory has been superseded by newer ones but is still used for the sake of demonstration. Clinical Application Transcutaneous Nerve Stimulation (TENS) is based on the Gate Control Theory. Nerves of the innocuous sensory system are stimulated and they in turn, inhibit transmission of nociceptive stimuli in the spinal cord. Abnormalities of Pain System Phantom Pain • Patients with amputation often have burning or tingling pain in the body part removed. • One possible cause is that nerve fibers at the stump are stimulated and the brain interprets the signals as originating in the amputated portion. • The other is the rearrangement within the cortical areas so that area say for the hand now responds to signals from other parts of the body but still interprets them as coming for the amputated hand. Somatosensory Cortex Organization Cortical Reorganization Brain surface of an owl monkey. The area of the hand representation for each of the 5 hand digits (1 to 5) After digit 3 was amputated, its area has been taken over by digits 2 and 4. Neuroscience. 2nd edition. Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Fig 25.14 Referred Pain • Often originates from a visceral organ. • May be felt in a part of the body remote from the site of the pathology. • The mechanisms may be spinal convergence of visceral and somatic afferent fibers on spinothalamic neurons. • Common manifestations: cutaneous and deep hyperalgesia, tenderness, muscular contractions. Pain sensation referred from visceral organs … … to another part of the body surface Purves et al. This talk was originally prepared by: Nilesh Patel, PhD University of Nairobi, Nairobi, Kenya International Pain School Talks in the International Pain School include the following: Physiology and pathophysiology of pain Nilesh Patel, PhD, Kenya Assessment of pain & taking a pain history Yohannes Woubished, M.D, Addis Ababa, Ethiopia Clinical pharmacology of analgesics and non-pharmacological treatments Ramani Vijayan, M.D. Kuala Lumpur, Malaysia Postoperative – low technology treatment methods Dominique Fletcher, M.D, Garches & Xavier Lassalle, RN, MSF, Paris, France Postoperative– high treatment technology methods Narinder Rawal, M.D. PhD, FRCA(Hon), Orebro, Sweden Cancer pain– low technology treatment methods Barbara Kleinmann, MD, Freiburg, Germany Cancer pain– high technology treatment methods Jamie Laubisch MD, Justin Baker MD, Doralina Anghelescu MD, Memphis, USA Palliative Care Jamie Laubisch MD, Justin Baker MD, Memphis, USA Neuropathic pain - low technology treatment methods Maija Haanpää, MD, Helsinki & Aki Hietaharju, Tampere, Finland Neuropathic pain – high technology treatment methods Maija Haanpää, M.D., Helsinki & Aki Hietaharju, M.D., Tampere, Finland Psychological aspects of managing pain Etleva Gjoni, Germany Special Management Challenges Debra Gordon, RN, DNP, FAAN, Seattle, USA International Pain School The project is supported by these organizations: