* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Taking fingerprints of stars
Stellar evolution wikipedia , lookup
Nucleosynthesis wikipedia , lookup
Planetary nebula wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Circular dichroism wikipedia , lookup
Microplasma wikipedia , lookup
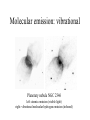
Taking the fingerprints of stars, galaxies, and interstellar gas clouds Absorption and emission from atoms, ions, and molecules The periodic table of the elements • The universe is mostly (97%) hydrogen and helium; H and He (and a little lithium, Li) were the only elements created in the Big Bang – heavier elements have all been (and are still being) manufactured in stars, via nuclear fusion • Each element has its own characteristic set of energies at which it absorbs or radiates The Bohr Atom • Hydrogen atom: consists of a proton (nucleus) “orbited” by an electron • Unlike a satellite, electron cannot “orbit” at arbitrary distances from nucleus – electron has specific, fixed set of “orbitals” • atomic structure is “quantized” • quantized structure first deduced by physicist Neils Bohr • Electron’s movement between orbitals requires absorption or radiation of energy – jump from lower to higher orbital: energy absorbed – jump from higher to lower orbital: energy emitted Bohr Atom: Extension to other elements • Although H is the simplest atom, the concept of electron orbitals applies to all elements • Neutral atoms have equal numbers of protons (in nucleus) and electrons (orbiting nucleus) – He has charge of 2; Li, 3; C, 6;etc... • The more electrons (protons) characterizing an element, the more complex its absorption/emission spectrum Absorption “lines” • First discovered in spectrum of Sun (by an imaging scientist named Fraunhofer) • Called “lines” because they appear as dark lines superimposed on the rainbow of the visible spectrum Sun’s Fraunhofer absorption lines (wavelengths listed in Angstroms; 1 A = 0.1 nm) Geometries for producing absorption lines The Observer • Absorption lines require a cool gas between the observer and a hot source – scenario 1: the atmosphere of a star – scenario 2: gas cloud between a star and the observer Emission line spectra Insert various emission line spectra here Geometries for producing emission lines The Observer • Emission lines just require a gas viewed against a colder background – scenario 1: the hot “corona” of a star – scenario 2: cold gas cloud seen against “empty” (colder) space The optical emission line spectrum of a young star Emission line images Green: oxygen; red: hydrogen Planetary nebula NGC 6543 (blue: Xrays) Orion Nebula Neon Iron Spectra of ions • Emission lines from heavy ions -- atoms stripped of one or more electrons -- dominate the high-energy (X-ray) spectra of stars • Ions of certain heavier elements (for example, highly ionized neon and iron) behave just like “supercharged” H and He Wavelength (in Angstroms) Molecular spectra • Molecules also produce characteristic spectra of emission and absorption lines – Each molecule has its particular set of allowed energies at which it absorbs or radiates • Molecules -- being more complex than atoms -- can exhibit very complex spectra – Electrons shared by one or more atoms in molecule will absorb or emit specific energies – Change in molecule’s state of vibration and/or rotation is also quantized • Vibration, rotation spectra unique to each molecule Molecular spectra (cont.) • Electronic transitions: mostly show up in the UV, optical, and IR • Vibrational transitions: mostly show up in the near-infrared • Rotational transitions: mostly show up in the radio Molecular emission: vibrational Planetary nebula NGC 2346 left: atomic emission (visible light) right: vibrational molecular hydrogen emission (infrared) Molecular emission: rotational Rotational CO (carbon monoxide) emission from molecular clouds in the Milky Way