* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 11 Notes
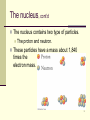
Survey
Document related concepts
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Isotopic labeling wikipedia , lookup
Muon-catalyzed fusion wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Technetium-99m wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Transcript
Chapter 11 Nuclear Physics The nucleus The nucleus occupies the very center of the atom. It is tiny yet incredibly dense: More than 99.9% of the atom’s mass is compressed into roughly one-trillionth of its total volume. The nucleus is impervious to the chemical and thermal processes that affect its electrons. 2 The nucleus, cont’d The nucleus contains two type of particles. The proton and neutron. These particles have a mass about 1,840 times the electron mass. 3 The nucleus, cont’d It is convenient to introduce an appropriate unit of mass, called the atomic mass unit, u: 1 u 1.66 10 27 kg 4 The nucleus, cont’d Recall that the number of protons in the nucleus is given by the atomic number, Z. This number identifies the type of atom. The neutrons play a smaller role in determining the properties of the atom. Their effect is mainly on the atom’s mass. The neutron number, N, is the number of neutrons contained in a nucleus. 5 The nucleus, cont’d The mass number, A, is the total number of protons and neutrons in a nucleus. AZ N We omit the electron’s mass because it so small compared to the proton’s and neutron’s. Protons and neutrons are collectively referred to as nucleons. 6 The nucleus, cont’d Each element has a given number of protons but can have different numbers of neutrons. Each different possible “type” of atom is called an isotope. Isotopes of a given element have the same number of protons in the nucleus but a different number of neutrons. Different isotopes have essentially the same atomic properties but different nuclear properties. 7 The nucleus, cont’d Most of the 114 different elements have several isotopes. Some have only a few: hydrogen has 3. Others have many: iodine, mercury and silver have more than 20. More than 2,500 different isotopes have been identified and studied. Only about 300 of these occur naturally. 8 The nucleus, cont’d The different isotopes of carbon are carbon-12, carbon-13, and carbon-14. The different isotopes of hydrogen have special names: hydrogen-2 is called deuterium. hydrogen-3 is called tritium. Isotopes play no role in chemical reactions. They are pivotal for understanding nuclear reactions. 9 The nucleus, cont’d We use a special notation to represent each isotope. The element’s chemical symbol is used. A subscript to the left of the chemical symbol represents the atom’s atomic number Z. A superscript to the left of the chemical symbol represents the atom’s atomic mass A. Helium-4 Carbon-12 4 2 12 6 He C Carbon-14 Uranium-235 14 6 235 92 C U 10 The nucleus, cont’d Notice that the subscript, the atomic number, must always agree with the chemical symbol. If the subscript is 6, the symbol must be for carbon, C. The number of neutrons can be found by subtracting the atomic number from the atomic mass: 235 92 U N 235 92 143 11 The nucleus, cont’d We use a similar notation for atomic particles. Proton 1 1 Electron 0 -1 n p e The superscript is the mass in atomic units. Neutron 1 0 Zero for the electron since it is so small. The subscript is the electric charge. 12 The nucleus, cont’d The nuclear strong force is responsible for binding protons in the nucleus against the electromagnetic force. Since protons are positively charged, they repel each other. At such short range, the electric force is tremendous. The nuclear force that “overpowers” the electric force was therefore given the name the strong force. 13 Radioactivity Radioactivity, also called radioactive decay, occurs when an unstable nucleus emits radiation. Isotopes with unstable nuclei are called radioisotopes. The majority of all isotopes are radioactive. 14 Radioactivity, cont’d This diagram shows the number of neutrons versus the number of protons in the isotopes. Stable isotopes are indicated by a small square. 15 Radioactivity, cont’d There are three types of nuclear radiation: alpha radiation (a) are made of helium nuclei. beta radiation (b) are made of high energy electrons. Two protons and two neutrons. Has a positive electric charge. Has a negative electric charge. gamma radiation (g) is EM radiation. Has no electric charge. 16 Radioactivity, cont’d The type of radiation can be determined by passing it through a magnetic field. Since each type has a different electric charge, they are deflected differently by the magnet. 17 Radioactivity, cont’d The most common type of radiation detector is the Geiger counter. The radiation ionizes the gas in a cylinder. The freed electrons are acceleration to the red wire and produce a current pulse. That pulse is counted. 18 Alpha decay Alpha decay occurs when an unstable nucleus ejects an alpha particle. Recall that an alpha particle is just a helium nucleus. two protons and two neutrons. alpha particle : a or 4 2 He The nucleus did not contain an alpha particle. This is just a stable collection of particles that can be ejected. 19 Alpha decay, cont’d The emission of an alpha particle: Reduces the number of particles by 4. The nuclear mass is reduced by 4 u. It reduces the number of protons by two, and It reduces the number of neutrons by two. A A 4 Z Z 2 N N 2 20 Alpha decay, cont’d Here is a diagram illustrating alpha decay. We start with an unstable plutonium nucleus. We obtain: a uranium nucleus, and an alpha particle. 21 Alpha decay, cont’d Alpha decay results in a drastic change in the mass of the nucleus. It typically occurs in radioisotopes with high atomic numbers. The ejected alpha particle is quickly absorbed by matter. A sheet of paper can stop it. 22 Example Example 11.1 The isotope radium-226 undergoes alpha decay. Write the reaction equations, and determine the identity of the daughter nucleus. 23 Example Example 11.1 ANSWER: The reaction is: 226 88 Ra He 4 2 226 4 88 2 ? He 222 86 ? He 222 86 Rn 4 2 4 2 24 Beta decay Beta decay occurs when an unstable nucleus ejects a beta particle. Recall that an beta particle is just an electron beta particle : b or 0 1 e The nucleus does not contain any electrons. A neutron is spontaneously converted into an electron and a proton. The electron is ejected at high speed while the proton remains in the nucleus. 25 Beta decay, cont’d The emission of an beta particle: Keeps the atomic mass the same but changes the type of nucleons. The nuclear mass is not changes. It increases the number of protons by one, and It reduces the number of neutrons by one. A A Z Z 1 N N 1 26 Beta decay, cont’d Here is a diagram illustrating beta decay. We start with a carbon-14 nucleus We obtain: a nitrogen-14 nucleus, and an beta particle (electron). 27 Beta decay, cont’d Beta decay results in (essentially) no change of the nuclear mass It is typically a rearranging of the type of nucleons toward a more stable configuration. The ejected beta particle passes easily through most matter. A sheet of lead provides a good shield against beta particles. 28 Example Example 11.2 The isotope iodine-131 undergoes beta decay. Write the reaction equations, and determine the identity of the daughter nucleus. 29 Example Example 11.2 ANSWER: The reaction is: 131 53 0 1 I e 131 54 ? e 131 54 Xe 0 1 30 Gamma decay Gamma decay occurs when an excited nucleus ejects a gamma particle. Recall that an gamma particle is just a photon in the gamma ray part of the EM spectrum. gamma particle : g The nucleus does not contain any photons. This decay is similar to an excited atom emitting a photon. 31 Gamma decay, cont’d The emission of an gamma particle: Keeps the atomic mass and atomic numbers the same. The nuclear mass is not changes. The number of protons remains the same. The number of neutrons remains the same. A A Z Z N N 32 Gamma decay, cont’d Here is a diagram illustrating gamma decay. We start with an excited strontium nucleus. We obtain: a strontium nucleus in a lower energy level, and an gamma particle. 33 Gamma decay, cont’d Gamma decay results in no change of the nuclear mass It is simple the emission of a photon due to the nucleus being in an excited state. The ejected gamma particle passes easily through almost all matter. A brick of lead provides a reasonably good shield against gamma particles. 34 Applications Nuclear medicine makes use of radioisotopes for diagnosis and treatment. Introducing a radioactive element into the blood stream allows the blood flow to be followed. Irradiating a tumor kills the tumor cells. Gamma radiation is very effective for sterilization. It kills virtually any organism it strikes. 35 Applications, cont’d Some smoke detectors use radioactive samples to monitor air particles. The sample ionizes the air which establishes a current. The ions attach to smoke particles and effectively reduce the current. The alarm then triggers. 36 Half-life Radioactive decay is a random process. An unstable isotope will decay but the exact amount of time until it decays is unknown. To overcome this, we talk about how much of a radioactive sample decays in a certain amount of time. Half-life is the time it takes for half the nuclei in a sample of a radioisotope to decay. The time interval during which each nucleus has a 50% probability of decaying. 37 Half-life, cont’d This table shows the half-life for several isotopes. Notice that the halflives range from extraordinarily short (2×10-21 s) to extremely long (4.5×109 yr). 38 Half-life, cont’d It is infeasible to count how many nuclei are left after a given time interval. But a Geiger counter can indicate how quickly radioisotopes are decaying. 39 Half-life, cont’d Knowing the half-life is very useful. Smoke detectors routinely use americium-241 since it has a half-life of 432 yrs. Enough time to allow for proper operation for the devices lifetime. Nuclear medicine uses technetium-99 since it emits gamma rays with a half-life of 6 hours. More than enough time to track its passage through the body. 40 Carbon dating The regular rate of decay of a radioisotope can be used to measure time. Carbon-14 dating uses the decay-rate of carbon-14 to determine how long ago an organism died. Carbon-14 is naturally created from nitrogen in the upper atmosphere. 41 Carbon dating, cont’d While a plant is alive, it absorbs carbon dioxide from the air. CO2 could be made from C-12, C-13 or C-14. Some animals eat the plant, while other animals eat the plant-eater. So each organism is continuously replenishing the amount of C-14 in its body. Once the organism dies, no more C-14 is consumed so the level of C-14 begins to decrease. 42 Carbon dating, cont’d This process can be used to determine how long ago an organism died. We know the average amount of C-14 in a living organism. We can measure the C-14 in a specimen. Comparing the values and knowing the halflife, gives information on how long ago it died. 43 Nuclear binding energy Imagine dismantling a nucleus by removing each proton and neutron, one at a time. Measure the amount of work required to remove each nucleon. The amount of energy required to assemble the nucleus equals the work required to disassemble it. This amount of energy is called the binding energy. 44 Nuclear binding energy, cont’d A convenient amount of energy is the amount of binding energy per nucleon. 45 Nuclear binding energy, cont’d Nuclei with mass numbers around 50 have the highest binding energy per nucleon. This means the protons and neutrons are more tightly bound to the nucleus. The nucleus is very stable. The idea of a nucleon being bound to the nucleus is similar to a ball resting in a hole. You have to do work on the ball to get it out of the hole. 46 Nuclear binding energy, cont’d If the nucleons are not tightly bound to the nucleus, a collision with another particle could split the nucleus. A neutron might impact uranium-235 to create barium-141 and krypton-92 (and three extra neutrons). Such a process is called nuclear fission. Energy is released during this process. 47 Nuclear binding energy, cont’d If two smaller nuclei collide, they might be able to increase their binding energy if they “stick” together. Hydrogen-1 and hydrogen-2 might combine to form helium-3. Such a process is called nuclear fusion. Energy is also released in this process. 48 Nuclear binding energy, cont’d Imagine combining a proton and a neutron. proton has 1.00785 u. neutron has 1.00869 u. After bonding, the combination has less mass than the two individual nucleons. 2.01410 u rather than 2.01654 u. 49 Nuclear binding energy, cont’d Einstein provided the reason for this: E mc 2 This formula means that energy and mass are different forms of the same quantity. Energy can be converted into mass. Mass can be converted into energy. This means that a hotter object (more energy) has more mass than the same object at a cooler temperature (less energy). 50 Nuclear binding energy, cont’d This difference in mass between individual nucleons and their combination, means we can liberate enormous amounts of energy. E mc 2 0.00244 u 3 10 m/s 4.0504 10 3.65 10 13 30 J. 8 2 kg 9 10 m /s 16 2 2 51 Nuclear fission Nuclear fission is the process by which nuclei split apart by absorbing a neutron. Fission is commonly accomplished by bombarding the radioisotope with neutrons. But alpha particles, gamma rays and protons have also proved successful. The resulting fragments are called fission fragments. 52 Nuclear fission, cont’d Two possible fission reactions of uranium-235 are: 1 0 n 235 92 U 236 92 141 56 1 0 n 235 92 U 236 92 140 54 U Ba Kr 3 n 92 36 U 1 0 Xe Sr 2 n 94 38 1 0 53 Nuclear fission, cont’d The amount of energy released during the fission of one U-235 nucleus is 215 million electron-Volts (about 3.4×10-11 joules). By comparison, the average energy involved in chemical process, e.g., metabolism in your body, is about 10 electron-Volts fro each molecule involved. 54 Nuclear fission, cont’d Two other important aspects of fission: The possible fission fragments are typically radioactive. The ratio of neutron to protons is too high for stability. These fragments are responsible for the radioactive fallout from nuclear explosions. The neutrons released by this process can cause fission in other nuclei. This process is called a chain reaction. 55 Nuclear fission, cont’d An atomic bomb can be created by increasing the density of radioisotopes so that a chain reaction begins. One way to accomplish this is to force together two pieces of uranium by an conventional explosive. You have a critical mass or U-235. 56 Nuclear fission, cont’d Another approach is to cause a single piece of radioactive sample to reach critical mass by compressing it. This type of bomb was dropped on Nagasaki. The other was dropped on Hiroshima. 57 Nuclear fission, cont’d Nuclear power plants need to be able to control the reaction rate so a chain reaction does not occur. First, a less-enriched sample of uranium is used. 58 Nuclear fission, cont’d Second, control rods are used to limit the number of neutrons that can participate in the fission process. 59 Nuclear fusion Nuclear fusion is the process of combining two nuclei to form a larger nucleus. Some common reactions are: H H H n 3.3 MeV 1 H H He 0 n 17.6 MeV 2 3 1 1 H 2 He He 1 p 18.3 MeV 2 1 2 1 2 1 3 1 3 2 4 2 4 2 1 0 60 Nuclear fusion, cont’d Energy is released in each case because the total mass of the nucleons after the fusion is less that the total mass before. 61 Nuclear fusion, cont’d Stars obtain most of their energy from a natural fusion reaction in the star’s interior. At the Sun’s core: the temperature is around 15 million degrees Celsius, and the pressure is over one billion atmospheres. These conditions force the hydrogen to fuse into helium. Each second, more than 4 million tons of matter are converted into energy. 62 Nuclear fusion, cont’d Thermonuclear weapons fuse hydrogen to generate their energy. To obtain the proper conditions for fusion, they use a nuclear fission explosion to trigger the fusion. A hydrogen bomb is to an atomic bomb what a stick of dynamite is to a firecracker. 63 Nuclear fusion, cont’d Controlled fusion is a technically challenging problem. The conditions which must be met are: The nuclei must be raised to extremely high temperature. The challenge here is to prevent such a hot material from contacting the confinement vessel. The must be sufficient density to maintain the fusion process. The plasma must be kept sufficiently dense so there are sufficient number of fusions. 64 Nuclear fusion, cont’d A current approach to accomplish this is through the use of a tokamak device. Electromagnets are used to confined the plasma. Other approaches use: laser beams to increase pressure; pulsed-power to “Z-pinch” a small pellet. 65