* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenitally corrected transposition of the great arteries: clues for
Survey
Document related concepts
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Artificial heart valve wikipedia , lookup
Cardiac surgery wikipedia , lookup
Electrocardiography wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Echocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Transcript
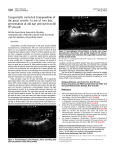
Ultrasound Obstet Gynecol 2004; 23: 68–72 Published online 27 November 2003 in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/uog.896 Congenitally corrected transposition of the great arteries: clues for prenatal diagnosis R. L. McEWING and R. CHAOUI Department of Obstetrics and Gynecology, Fetal Medicine Unit, Charité Hospital, CCM, Humboldt University, Berlin, Germany K E Y W O R D S: atrioventricular discordance; corrected transposition; fetal echocardiography ABSTRACT Congenitally corrected transposition of the great arteries (ccTGA) is an uncommon cardiac defect characterized by the atria connecting with the anatomically discordant ventricles and the ventricles connecting with discordant and transposed great arteries. Parallel vessels are evident in corrected TGA, but as this sign is also present in complete TGA, a heart anomaly requiring major cardiac surgery in the postnatal period, it is important to differentiate between the entities prenatally. Most cases of ccTGA have associated anomalies but isolated forms or those with a mild associated cardiac anomaly are infrequently detected prenatally. We report on three cases detected between 21 and 25 weeks’ gestation on screening ultrasound with associated mild findings. One fetus had an isolated ventricular septal defect (VSD) first detected at 34 weeks. The child developed heart block at 4 years of age. The second case was associated with a small VSD, a tiny pulmonary trunk and a persistent right umbilical vein. After birth, mild pulmonary stenosis was found as an additional cardiac finding at 4 months of age. The third fetus had no additional cardiac anomalies prenatally, but after birth a bicuspid aortic valve was detected. The first case needed pacemaker implantation but the other two children required no cardiac surgery. Two of the cases were referred because abnormal vessel anatomy was detected on screening ultrasound. As prenatal detection of TGA is becoming a more frequent occurrence, this paper aims to present clues aiding in the prenatal diagnosis of atrioventricular and ventriculoarterial discordance, especially in its differentiation from complete transposition. These details are crucial for counseling and perinatal management. Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. INTRODUCTION Congenitally corrected transposition of the great arteries (ccTGA) is an uncommon congenital cardiac anomaly, accounting for less than 1% of live births with congenital heart disease. It is characterized by normal veno-atrial connections, but with discordant atrioventricular and ventriculoarterial connections, allowing hemodynamic compensation (Figure 1). Isolated cases and those with mild anomalies are rarely detected in the neonate. There are generally associated cardiac anomalies that influence the prognosis. Parallel vessels are evident in ccTGA, but as this sign is also present in the more common complete TGA (d-TGA) (Figure 1), a heart anomaly requiring major cardiac surgery in the neonatal period, it is important to differentiate between the entities prenatally. Careful examination of the ventricles allows the morphological right and left ventricles to be distinguished and atrioventricular discordance to be detected (Figures 2–5). We describe the prenatal sonographic characteristics and neonatal course of three cases of essentially isolated corrected transposition. CASE REPORTS Case 1 A 31-year-old woman was referred at 25 weeks’ gestation due to inadequate visualization of the four-chamber heart at another institution. Detailed scanning demonstrated normal growth, no anomalies and a normal four-chamber view; however, the great vessels were inadequately evaluated. The patient returned 2 weeks later, at 27 weeks’ gestation, for targeted fetal echocardiography. Two-dimensional (2D) and color Doppler ultrasound revealed normal abdominal and thoracic situs, but atrioventricular discordance in the four-chamber view. The anatomic right atrium connected to a right-sided morphologic left ventricle, and the left atrium connected to a left-sided morphologic right ventricle. Ventriculoarterial discordance (with levo-transposition) of the great vessels was evident with the pulmonary trunk arising from the morphologic left ventricle (situated on the right side) and Correspondence to: Prof. R. Chaoui, Department of Obstetrics and Gynecology, Charité Medical School, Humboldt University, Schumannstrasse 20/21, D-10098 Berlin, Germany (e-mail: [email protected]) Accepted: 20 August 2003 Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. CASE REPORT Prenatal diagnosis of corrected transposition SVC 69 TP Ao LA RA TP Ao LA RA RV RV LV LV IVC Complete transposition (d-TGA) Corrected transposition Figure 1 Schemes comparing complete and corrected transposition of the great arteries. In complete transposition the ventricles are normally connected (RA with RV, LA with LV), whereas in corrected transposition there are discordant connections (RA with LV, LA with RV). Note that the right-sided ventricle has a left morphology without trabeculation and reaches the heart apex. In both cases vessels are arising from the ‘wrong’ ventricle and have a parallel course. In complete TGA the aorta lies to the right of the pulmonary trunk (d-looping), and in the corrected form to the left (l-looping). In complete TGA in postnatal life, deoxygenated blood arriving in the right atrium will enter the right ventricle and thence the aorta without passing through the lung (causing cyanosis). In corrected TGA, blood entering the anatomical left ventricle and then the pulmonary trunk is oxygenated (therefore ‘congenitally corrected’). RA, LA, right and left atria; RV, LV, right and left ventricles; Ao, aorta; TP, pulmonary trunk; IVC, SVC, inferior and superior venae cavae. the aorta arising from the morphologic right ventricle (situated on the left side). The vessels had a parallel course, with the aorta lying ventrally and to the left of the pulmonary trunk (l-transposition). There were no additional anomalies or arrhythmia. The diagnosis of isolated ccTGA was made. The patient declined karyotyping. A scan at 32 weeks confirmed these findings, and color Doppler aided in additional identification of a muscular ventricular septal defect (VSD) by showing bidirectional shunting. Later sonograms showed intrauterine growth restriction with normal fetal Doppler indices and normal cardiac rhythm. A male infant weighing 2890 g was delivered spontaneously at 38 weeks’ gestation at our center. Postnatal echocardiography confirmed the prenatal findings. The newborn was discharged at 6 days of age. By age 12 months, echocardiography demonstrated spontaneous closure of the VSD. Later echocardiographic examinations showed normal ventricular contractility until 4 years of age when intermittent bradycardia became apparent. Twenty-four hour electrocardiogram (ECG) monitoring revealed an intermittent atrioventricular block with a ventricular rate of 55 bpm, necessitating pacemaker insertion at 5 years of age. The child remains well at 6 years of age, with good cardiac function. consultation was to plan optimal neonatal management and surgery, as the patient lived 250 km from our center. At targeted fetal echocardiography, utilizing 2D and color Doppler, there was normal abdominal and thoracic situs, but ccTGA was evident (as in the description of Case 1). Targeted examination with color Doppler revealed an additional small (2 mm) subpulmonic VSD. The pulmonary trunk had a smaller caliber than the aorta (3.4 vs. 4.1 mm), suggesting possible pulmonary stenosis, but peak velocity across the pulmonary valve was 105 cm/s, within the normal range, with no turbulent flow. No arrhythmia was documented during the course of the pregnancy. There was persistence of the right umbilical vein, coursing to the right of the gallbladder towards the normally developed ductus venosus and portal system. The patient was counseled as for a compensated cardiac malformation with no necessity for a switch operation after birth. A repeat ultrasound scan at 25 weeks confirmed the findings of ccTGA with VSD, but demonstrated absence of a pressure gradient across the pulmonary valve. Accidental preterm home birth occurred at 31 weeks (a male infant weighing 1660 g), and the infant was subsequently admitted to the neonatal intensive care unit at the local hospital. The neonatal course was uneventful, with cardiac compensation, and the child was discharged 6 weeks later, weighing 2540 g. Postnatal echocardiography confirmed the prenatal diagnosis of ccTGA, with a 4-mm VSD. The great vessels were of normal size and normal pressure. Echocardiography at age 4 months demonstrated subvalvular and valvular pulmonary stenosis with a pressure gradient of 64 mmHg, in addition to the ccTGA and VSD. The child is now developing normally at 3 years of age, and echocardiography and ECG are stable, with no evidence of arrhythmia or signs of cardiac decompensation. Case 3 Fetal echocardiography at 23 weeks’ gestation incidentally revealed ccTGA, with normal abdominal and thoracic situs, in a 17-year-old patient who was a volunteer at an obstetric ultrasound course. No additional heart defects or arrhythmia were detected. The karyotype was that of a normal male, with no 22q11.2 deletion. A male infant (3060 g) was delivered spontaneously at 38 weeks. Postnatally, intravenous prostaglandin was initiated, due to the ‘TGA’ diagnosis, but was discontinued when echocardiography confirmed ccTGA. Additionally, there was a bicuspid aortic valve with normal velocities across the valve. The child had a satisfactory neonatal course and was discharged at 3 days. He is now 1 year old with no arrhythmia or additional cardiac findings demonstrated on the most recent echocardiography and ECG. Case 2 DISCUSSION A 21-year-old patient was referred at 20 weeks for a second opinion scan after the diagnosis of complete TGA was made at screening ultrasound. The aim of the ccTGA is an uncommon congenital cardiac anomaly, characterized by atrioventricular and ventriculoarterial discordance1 . In this condition, with solitus (normal) Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. Ultrasound Obstet Gynecol 2004; 23: 68–72. 70 McEwing and Chaoui Figure 2 Sonographic appearances of complete transposition of the great arteries. In atrioventricular concordance the tricuspid valve attaches lower within the ventricle, there is a moderator band on the right side and the lumen of the right ventricle is shorter than the left. The great vessels arise in a parallel course and the aorta lies to the right and anterior of the pulmonary artery. AO, aorta; MV, mitral valve; RA, LA, right and left atria; RV, LV, right and left ventricles; TP, pulmonary trunk; TV, tricuspid valve. atrial arrangement, the systemic veins drain into the morphologic right atrium, which is connected to the morphologic left ventricle by a mitral valve. The pulmonary artery is discordantly connected and transposed, arising from the left ventricle. The left atrium, receiving the pulmonary veins, joins the morphologic right ventricle via a tricuspid valve and the transposed aorta arises from the right ventricle. The aorta is located anteriorly and to the left of the pulmonary trunk (Figure 6). Transposition of the great vessels theoretically corrects for the hemodynamic effects of the ventricular inversion. However, associated cardiac anomalies are common, and include cardiac malpositioning and situs inversus, tricuspid valve dysplasia or atresia, double outlet ventricle, VSD (present in over 50% of cases and usually large and perimembranous) and pulmonary outflow tract obstruction (in about 50% of cases, and frequently associated with a large VSD)1 – 6 . Abnormal atrioventricular conduction tissue frequently causes conduction disturbances and predisposes to spontaneous heart block1 – 6 . The estimated prevalence is less than 1% of live births with congenital heart disease; but this estimate may be misleading as in utero deaths from associated severe anomalies, particularly tricuspid valve dysplasia or displacement, are not uncommon1 – 3,5 . Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. Embryologically, the condition is believed to be due to abnormal left-looping (l-ventricular looping) of the primitive cardiac tube, connected at one end to the sinus venosus and at the other to the truncus arteriosus. The morphologic right ventricle becomes left-sided, the morphologic left ventricle becomes relatively rightsided, and the interventricular septum may become more horizontally aligned, due to relative supero-inferior positioning of the ventricles. The conotruncal septum fails to rotate in the usual spiral fashion, leading to a parallel arrangement of the outflow tracts. The mitral and pulmonary valves are in fibrous continuity, while the tricuspid valve is separated from the aortic valve by a complete right ventricular infundibulum. The aorta frequently lies anteriorly and to the left of the pulmonary trunk1 – 3 . In the absence of associated anomalies, patients may be initially asymptomatic, with presentation not uncommonly in adult life with conduction disturbance, atrioventricular valve regurgitation or impairment of systemic ventricular function and congestive heart failure5,6 . With associated cardiac anomalies, presentation in the neonatal period with bradycardia from atrioventricular block or congestive heart failure is common, the latter often attributable to associated VSD, tricuspid valve dysplasia or displacement or aortic arch obstruction1,2 . A major Ultrasound Obstet Gynecol 2004; 23: 68–72. Prenatal diagnosis of corrected transposition 71 Figure 3 Sonographic appearances of corrected transposition of the great arteries. In atrioventricular discordance the right ventricle lies on the left side and vice versa. The tricuspid valve is thus on the left side, attaching lower within the ventricle, there is a moderator band on the left side and the lumen on the left side is shorter than the right. MV, mitral valve; RA, LA, right and left atria; RV, LV, right and left ventricles; TV, tricuspid valve. LV LA RA RV LA RA LV RV Normal (AV-concordance) Figure 5 Attachment of the papillary muscles in corrected transposition of the great arteries. The vertical arrow points to the attachment of the papillary muscles on the free wall and therefore it is a mitral valve and the ventricle is the left ventricle. The horizontal arrows point to the papillary attachment at the septum and the apex, evidence of a tricuspid valve and an anatomically right ventricle. RA, LA, right and left atria; RV, LV, right and left ventricles. Corrected TGA (AV-discordance) Figure 4 Attachment of the papillary muscles differs for the tricuspid and mitral valves. The mitral valve papillary muscles (black arrow) attach to the free wall and the tricuspid valve papillary muscles (arrow head) attach to the heart apex and septum as demonstrated in the left image demonstrating atrioventricular concordance (the moderator band at the apex of the right ventricle is indicated by a white arrow). In atrioventricular discordance, the mitral valve is on the right side, the papillary muscles attach to the free wall, and the tricuspid valve papillary muscles attach to the apex on the left (compare with Figure 5). AV, atrioventricular; RA, LA, right and left atria; RV, LV, right and left ventricles; TGA, transposition of the great arteries. concern in long-term follow-up in patients with ccTGA is, however, the unpredictability of the right ventricle to sustain systemic pressure. The development of tricuspid regurgitation is considered a risk factor and sign of developing cardiac failure, which may lead to death or necessitate cardiac transplantation5 . Prenatal diagnosis is difficult and has been infrequently reported. As far as we are aware, no reports exist Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. in the literature to date of prenatal diagnosis of isolated ccTGA. Associated anomalies, such as systemic ventricular hypoplasia, severe aortic coarctation4 , situs inversus7 , twisted atrioventricular connections8,9 and complete heart block2 , have been described in the few cases of ccTGA within the prenatal literature. Fetal echocardiography should focus on identification of the morphologic left and right ventricles. The fourchamber view allows assessment of typical ventricular morphology; the morphologic right ventricle is characterized by a prominent moderator band, more apical attachment of the atrioventricular valve, chordal attachment of the atrioventricular valve directly to the septum, irregularity of the endocardial surface compared to the smooth surface of the left ventricle and a more rounded or triangular shape to the cavity compared to the more elongated appearance of the morphological left ventricle1,2 . The papillary muscles of the morphological right ventricle tend to attach distally and centrally, in contradistinction to those of the morphological left ventricle, which attach to the side wall of the ventricle. In congenitally corrected transposition of the great vessels, the morphological right ventricle lies posteriorly and to the left of the morphological left ventricle. There may be malalignment of the interatrial septum relative to the interventricular septum1 . Associated malformations may be evident on the fourchamber view; in particular VSD or Ebstein’s anomaly Ultrasound Obstet Gynecol 2004; 23: 68–72. McEwing and Chaoui 72 to hemodynamic changes occurring after birth, and the possible development of heart block or right ventricular dysfunction at any stage2 . With detection of additional cardiac anomalies, the prognosis depends on the type of associated abnormality. In conclusion, the detection of parallel vessels on prenatal ultrasound arising from two different chambers is most likely to be complete transposition, but careful examination of the chamber anatomy in the four-chamber plane, in addition to assessment of the connection and position of the great vessels (i.e. d- vs. l-malposition), helps in the prenatal differentiation of ccTGA. REFERENCES Figure 6 Identification of a parallel course of the great vessels, with the aorta anteriorly and to the left of the pulmonary trunk, completes the diagnosis of corrected transposition of the great arteries. AO, aorta; RV, LV, right and left ventricles; TP, pulmonary trunk. of the left atrioventricular valve should be excluded. The outflow tracts should be carefully assessed; the two great vessels characteristically arise with a parallel course. The pulmonary outflow tract, arising from the morphological left ventricle, is embedded between the two atrioventricular valves10 . Color Doppler facilitates assessment of the course of the great vessels and associated anomalies such as atrioventricular valvular regurgitation and the bidirectional shunt of VSD. Pulsed or continuous wave Doppler may demonstrate an associated stenosis of the outflow tracts, most commonly the pulmonary trunk. Hydrops may be evident in fetuses with associated cardiac anomalies. Heart block is uncommonly documented prenatally, but has been reported1,2,4 . In prenatally diagnosed patients without associated anomalies, a good mid-term prognosis may be expected, but prediction of long-term prognosis can be difficult due Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd. 1. Freedom RM, Dyck JD. Congenitally corrected transposition of the great arteries. In Moss and Adams Heart Disease in Infants, Children, and Adolescents Including the Fetus and Young Adult (5th edn), Emmanouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP (eds). Williams & Wilkins: Baltimore, MD, 1995; 1225–1242. 2. Stewart PA, Wladimiroff JW. Ventricles, inverted with transposition of the great arteries. 1993. http://www.TheFetus.net [Accessed 7 July 2003]. 3. Freedom RM, Benson LN. Congenitally corrected transposition of the great arteries. In Neonatal Heart Disease, Freedom RM, Benson LN, Smallhorn JF (eds). Springer-Verlag: London, Berlin, New York, 1992; 523–542. 4. Santoro G, Masiello P, Baldi C, Farina R, Fittipaldi O, Di Benedetto G. Corrected transposition of the great arteries with isolated aortic coarctation: in utero echocardiographic diagnosis. Pediatr Cardiol 1997; 18: 396–398. 5. Rutledge JM, Nihil MR, Fraser CD, O’Brien Smith E, McMahon CJ, Bezold LI. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol 2002; 23: 137–145. 6. Presbitero P, Somerville J, Rabajoli F, Stone S, Conte MR. Corrected transposition of the great arteries without associated defects in adult patients: clinical profile and follow up. Br Heart J 1995; 74: 57–59. 7. Carvalho JS, Kyle PM. Situs inversus with complete transposition in the fetus. Circulation 1997; 96: 4432–4433. 8. Morelli PJ, Kimball TR, Witt SA, Meyer RA. Echocardiographic considerations in demonstrating complex anatomy of criss-cross atrioventricular valves and discordant atrioventricular and ventriculoarterial relations. J Am Soc Echocardiogr 1996; 9: 727–729. 9. Abdullah M, Yoo S-J, Hornberger L. Fetal echocardiographic features of twisted atrioventricular connections. Cardiol Young 2000; 10: 409–412. 10. Allan L. Atrioventricular discordance. In Textbook of Fetal Cardiology, Allan L, Hornberger L, Sharland G (eds). Greenwich Medical Media Limited: London, UK, 2000; 183–192. Ultrasound Obstet Gynecol 2004; 23: 68–72.