* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HIV
Survey
Document related concepts
Transcript
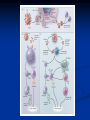
HIV Antiretroviral Treatment By: Richard Britt Dr. Buynak Spring 2006 Human Immunodeficiency Virus HIV is a Retrovirus which means: It contains a single-stranded RNA genome The HIV will incorporate it’s own genome into it’s host cell and hijack the normal functions of the cell to replicate itself This process will eventually lead to cell destruction The target for HIV is the CD-4+ Helper TCells, which are the backbone of the immune system. Symptoms The Majority of Symptoms of an HIV infection do not show up until the disease has already begun to damage the immune system The incubation time for an HIV infection can be several weeks to several years General symptoms include: Lack of energy, weight loss, frequent fevers and sweats, persistent or frequent yeast infections, persistent skin rashes or flakey skin, short-term memory loss, and mouth, genital, or anal sores from Herpes infections Opportunistic Infections HIV infection is usually discovered when a patient is diagnosed with an unusually severe or persistent infection Opportunistic infections include: Bacterial, Fungal, Parasitic, and Viral Infections These infections will be more severe because the person’s immune system will be surpressed by the HIV disease. HIV Details RNA Genome is 9 kilobases long and contains 9 genes that encode 15 different protiens Fusion targets of the viral surface envelope glycoprotiens is the CD4+ receptor and its coreceptor CCR5 on the surface of the Tlymphocyte Envelope contains the following viral enzymes: Reverse Transcriptase, Integrase, RNAse-H Basic Steps HIV fuses with host cell and releases its genome and enzymes into the cell RNA genome is transcribed by Reverse Transcriptase into a single stranded viral DNA Reverse Transcriptase acts as DNA Polymerase and transcribes the single stranded DNA into a Double Stranded Viral DNA DNA is then transported into the cell nucleus and is integrated into the host cell DNA by the viral enzyme integrase. HIV Lifecycle More Basic Steps Normal functions of the cell resume except now instead of transcribing RNA for the regular proteins of the cell it is transcribing viral mRNA Viral Proteins are produced in one large multiprotein chain from the viral mRNA Viral Components move toward the cell membrane and bud off into new immature virions HIV Lifecycle More Basic Steps Viral enzyme protease cleaves itself from the viral protein mass The Viral Protease then matures the virion by cutting up the protein mass into the individual viral enzymes The virion is a mature and infectious virus HIV Lifecycle Mature HIV Virus Life Cycle Animation Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Zidovudine first drug in this class approved by the FDA on March 20, 1987 This class of drugs works by inhibiting the action of the viral enzyme reverse transcriptase. This is accomplished by taking the place of a DNA peptide and prematurely terminating the transcription process NRTIs are phosphorylated three times after they enter the cell to become successful inhibitors Zidovudine (AZT or azidothymidine) Nucleotide Reverse Transcriptase Inhibitors (NtRTIs) In the same class of drugs as NRTIs however these are not required to be phosphorylated after they enter the cell. Same mechanism of action as NTRIs Life Cycle Animation Tenofovir Disproxil Fumarate (Viread®) Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) Also act as inhibitors of the viral enzyme reverse transcriptase however mechanism of action is different This class of drugs works by non-competitive inhibition The drug binds to the viral enzyme at a place other than the active site and changes the conformation of the active site decreasing the enzyme’s affinity for nucleoside binding. Nevirapine (Viramune®) Life Cycle Animation Protease Inhibitors These work by competitive inhibition of the viral enzyme protease These drugs irreversibly bind to the active site of protease preventing it from completing the maturation of the virion Protease inhibitors prevent immature virions from becoming mature, infectious Viruses Life Cycle Animation Sequinavir (Invirase®) Low Bioavailability Ritonvir (Norvir®) More successful because it inhibits Cytochrome P450 3A4 which breaks down Protease Inhibitors Mature HIV Virus Life Cycle Animation Fusion Inhibitors Newest Class of Drugs This drug binds to the glycoprotein gp41 in the viral envelope inhibiting its fusion with the CD4+ receptor on the host cell and thus preventing the cell’s infection. Usually used as a last line Enfuvirtide (Fuzeon®) option for most patient because it is only available as an injection and its high cost Life Cycle Animation More than $25000 per year Fusion Inhibitors vs. Other Classes of Drugs History of Drug Development 1985 – research on anti-viral medication begins 1987 – First drug Zidovudine produced Mid-1990s – Protease Inhibitors and NNRTIs Developed First NRTI Early life extending properties except only temporarily worked as patients became immune 1995 – first protease inhibitor Sequinavir approved by the FDA Low Bioavailability led to the development of a second protease inhibitor Ritonvir 1996 first NNRTI, Nevirapine approved by FDA March 2003 – First Fusion Inhibitor Enfuvirtide approve by FDA Current Research HIV Vaccine Two methods of vaccine research showing promise Recombinant Subunit Vaccines Live Recombinant Vaccines Disadvantages Recombinant Subunit Vaccine If the recombinant viral envelope proteins that are included in the vaccine aren’t close enough in structure and composition to the actual HIV envelope proteins the antigens that are produced will be ineffective in fighting an actual HIV infection Live Recombinant Vaccine Can actually cause disease when it is trying to prevent it Usually occurs when the person is immunocompromised Sources Cervia, Joseph S. and Miriam A. Smith. “Enfuvirtide (T-20): A Novel Human Immunodeficiency Virus Type 1 Fusion Inhibitor.” Clinical Infectious Diseases 2003; 37:1102-1106. NATAP.ORG. 5 April 2005. <http://www.natap.org/2003/oct/100703_1.htm>. Greenberg, Michael L. and Nick Cammack. “Resistance to enfuvirtide, the first HIV fusion inhibitor.” The Journal of Antimicrobial Chemotherapy. 2004 54(2):333-340. Oxford Journals; British Society for Antimicrobial Chemotherapy: 2004. 5 April 2006. <http://jac.oxfordjournals.org/cgi/content/full/54/2/333>. “HIV/AIDS.” eMedicine Consumer Health. WebMD: 2003-2006. 5 April 2006. <http://www.emedicinehealth.com/hivaids/article_em.htm>. “HIV Fusion Inhibitor.” Panacos. Panacos Pharmaceuticals Inc.: 2006. 5 April 2006. <http://www.panacos.com/product_3.htm>. “HIV Lifecycle” Roche-HIV.com. F. Hoffmann-La Roche Ltd.: 2006. 5 April 2006. <http://www.rochehiv.com/Newsandfeatures/animations/lifecycle/lifecycle_animation.cfm?link=HIVTreatment#> “Maenza, Janine, and Charles Flexner. "Combination antiretroviral therapy for HIV infection." American Family Physician 57.n11 (June 1998): 2789(10). InfoTrac OneFile. Thomson Gale. Southern Methodist University. 5 April 2006 <http://find.galegroup.com/itx/infomark.do?&contentSet=IACDocuments&type=retrieve&tabID=T002&prodId=ITOF&docId=A20881343&source=gale&userGroupName=txshracd2548&version=1.0>. Peiperl, Laurence, Susa Coffey, and Paul Volberding. “HIV InSite Knowledge Base.” Comprehensive on-line textbook of HIV disease from the University of California San Francisco and San Francisco General Hospital. Regents of the University of California: 2006. 5 April 2006. <http://hivinsite.ucsf.edu/InSite.jsp?page=KB>. Phillips, Kenneth D. "Protease inhibitors: a new weapon and a new strategy against HIV." Journal of the Association of Nurses in AIDS Care 7.n5 (Sept-Oct 1996): 57(15). InfoTrac OneFile. Thomson Gale. Southern Methodist University. 5 April 2006 <http://find.galegroup.com/itx/infomark.do?&contentSet=IACDocuments&type=retrieve&tabID=T002&prodId=ITOF&docId=A18944501&source=gale&userGroupName=txshracd2548&version=1.0>. “Primate Lentivirus Group: HIV-1, HIV-2.” Daniele Focosi. 2001-2005. 5 April 2006. <http://focosi.altervista.org/pathoviruses_HIV.html> Reisberg, Paul. “HIV Part 1.” Wellesley Chemistry Department: March 8, 1999. 5 April 2006. <http://www.wellesley.edu/Chemistry/Chem101/hiv/HIV1.html> “Simple Facts Project.” AIDS Treatment Data Network. The Network: 2002. 5 April 2005. <http://www.aegis.com/factshts/network/sf.html>. Stanic, Anela, and Tulip K. Schneider. "Overview of antiretroviral agents in 2005." Journal of Pharmacy Practice 18.4 (August 2005): 228. InfoTrac OneFile. Thomson Gale. Southern Methodist University. 07 February 2006 <http://find.galegroup.com/itx/infomark.do?&contentSet=IACDocuments&type=retrieve&tabID=T002&prodId=ITOF&docId=A137362742&source=gale&srcprod=ITOF&userGroupName=txshracd2548&version =1.0>. Zapor, Michael J., Cozza, Kelly L., et al. “Antiretrovirals, Part II: Focus on Non-Protease Inhibitor Antiretrovirals (NRTIs, NNRTIs, and Fusion Inhibitors).” Psychosomatics; 45:6 (Nov – Dec 2004): 524-535. 5. April 2006 <http://psy.psychiatryonline.org/cgi/reprint/45/6/524>.