* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download cholesterol and lipo..
Survey
Document related concepts
Peptide synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Human digestive system wikipedia , lookup
Transcript
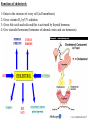
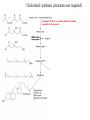
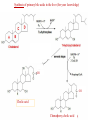
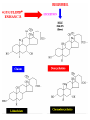
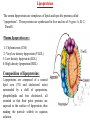
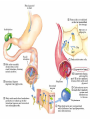
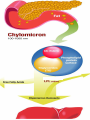
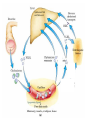
Cholesterol Sources: Exogenous: from diet, it is present in egg yolk, liver and brain. Endogenous: synthesized in all cells of the body from acetyl CoA. e.g. in liver (mainly), intestine, adrenal cortex, ovaries, testes and skin. Digestion and absorption: mainly in intestine Most dietary cholesterol is present in free form (not estrified) with 10-15% present as ester (fatty acid attached to OH at C3). Cholesterol esterase secreted in pancreatic juice acts on cholesterol ester giving free cholesterol and fatty acids. Bile acids are necessary for activation of this enzyme. Cholesterol In intestinal mucosa, the major amounts of free cholesterol (about 80-90%) combine with acyl CoA (active form of fatty acids) to form cholesterol esters which transported in blood with TAG, phospholipids and apoprotein to form lipoprotein chylomicron. The enzyme that catalyzes cholesterol esterification in mucosa is ACAT (Acyl CoA: Cholesterol Acyl Transferase). Functions of cholesterol: 1- Enter in the structure of every cell (cell membrane). 2- Gives vitamin D3 by UV radiation 3- Gives bile acids and salts and this is activated by thyroid hormone. 4- Give steroidal hormones (hormones of adrenal cortex and sex hormones). Synthesis: About 50% of body cholesterol is derived from de novo biosynthesis. De novo synthesis of cholesterol occurs mainly in cytoplasm of liver and intestine from acetyl CoA derived from oxidation of glucose and fatty acids in mitochondrial matrix, so acetyl CoA must be transported from mitochondria to cytoplasm as citrate (as in fatty acid synthesis). Transport of acetyl CoA from mitochondria to cytoplasm: Mitochondria: OAA + Acetyl CoA -CoA ↓ citrate synthase Citrate Inner mitochondrial membrane ↓ Citrate + CoA, ATP ↓ ATP citrate lyase Cystosol OAA + Acetyl CoA The process of cholesterol synthesis has five major steps: 1. Three molecules of Acetyl-CoAs are converted to HMG-CoA 2. HMG-CoA is converted to mevalonate 3. Mevalonate is converted to the isoprene based molecule. 4. Isoprene is converted to squalene 5. Squalene is converted to cholesterol Cholesterol synthesis (structures not required) cytoplasmic H MG-CoA synthase (different from that responsible for ketogenesis) Regulation of cholesterol synthesis: HMG- CoA reductase is the key (rate limiting) enzyme in the biosynthesis of cholesterol. It is regulated by: 1- Feed back inhibition by cholesterol: Cholesterol (the end product of the pathway) acts as feed back inhibitor of the pre-existing HMG –CoA reductase as well as inducing rapid degradation of the enzyme.. 2- Drug inhibition: Statins such as atorvastatin (by Pfizer), lovastatin and simvastatin are drugs with a side chain structurally similar to HMG-CoA so competitively inhibit HMG-CoA reductase. They are used to decrease cholesterol levels in patients with hypercholesterolemia. 3- Diet: its activity activated by high CHO and fat diet and inhibited in starvation. Catabolism of cholesterol: 80-90% of cholesterol is converted (oxidized) into bile acids then to bile salts. This is activated by thyroxin hormone. Bile acids: Two bile acids are synthesized in liver from cholesterol, these are: cholic acid and chenodeoxy cholic acid . chenodeoxycholic acid and cholic acid are called primary bile acids. Within the intestines the primary bile acids are converted by intestinal bacteria to the secondary bile acids, identified as deoxycholic acid and lithocholic acid. Both primary and secondary bile acids are reabsorbed by the intestines and go back to the liver via the portal circulation for the formation of bile salts in liver. Synthesis of primary bile acids in the liver (for your knowledge) OH OH OH Cholic acid Chenodeoxy cholic acid Formation of bile salts: Cholic acid is conjugated with either glycine or taurine forming glycocholic acid or taurocholic acid. Bile salts are Na+ or K+ salts of glycocholate or taurocholate. Bile salts are carried from the liver to gallbladder, where they are stored for future use. Functions of bile salts in fat metabolism: 1. their synthesis and subsequent excretion in the feces represent the only significant mechanism for the elimination of excess cholesterol. 2. Bile salts and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder. 3. they facilitate the digestion of dietary triacylglycerols by acting as emulsifying agents that render fats accessible to pancreatic lipases. 4. Activate steapsin and cholesterol esterase Disturbances in cholesterol metabolism: Plasma cholesterol: normal cholesterol level in blood is 150-250 mg/dL (average 200 mg/dL). Plasma cholesterol is derived mainly from liver and intestine. Hypercholesterolemia: i.e increased cholesterol level in blood than 250 mg/dL: Causes: 1- Hypothyrodism: lead to decreased conversion of cholesterol into bile acids and decreased mobilization of cholesterol from blood to tissues. 2- Obstructive jaundice: because the blocking of bile duct → ↓ excretion of cholesterol through intestine with feces. 3- Diet rich in carbohydrates, and fats: increase the synthesis of cholesterol in liver due to: a - Increased the activity of the key enzyme (HMG-CoA reductase). b - Formation of excess acetyl CoA (from fats and CHO) than the requirements of kreb’s cycle. 4- High cholesterol in diet Hypocholesterolemia: decrease of cholesterol level in blood below 150 mg/dL: 1- Hyperthyrodism: ↑ thyroid hormone→ a- ↑ oxidation of cholesterol into bile salts. b- increase mobilization of cholesterol from blood to tissues. 2- Liver disease: leads to decreased cholesterol synthesis which occurs in liver 3- Starvation: inhibits cholesterol synthesis by decreasing HMG-CoA reductase. 4- Estrogens: decrease cholesterol and prevent atherosclerosis, so, coronary heart disease rarely occurs in females during reproductive period of life. Study question: High carbohydrate diet may lead to all of the following EXCEPT: a) Ketosis due to stimulation of HMG-CoA synthase b) Hypercholesterolemia due to stimulation of HMGCoA reductase c) Over synthesis of neutral fat (TAG) d) Increased synthesis of acetyl CoA carboxylase Lipoproteins The serum lipoproteins are complexes of lipids and specific proteins called "apoproteins". These proteins are synthesized in liver and are of 5 types: A, B, C, D and E. Plasma lipoproteins are: 1- Chylomicrons (CM) 2- Very low density lipoprotein (VLDL) 3- Low density lipoprotein (LDL) 4- High density lipoprotein (HDL) Composition of lipoproteins: Lipoproteins are composed of a neutral lipid core (TG and cholesterol ester) surrounded by a shell of apoproteins, phospholipids and free cholesterol, all oriented so that their polar proteins are exposed to the surface of lipoprotein, thus making the particle soluble in aqueous solution. Functions of Lipoproteins Help to transport lipids in blood. Since lipids are water insoluble, they can't be transported in blood. For their transport, they are conjugated with apoprotiens forming lipoproteins which are water soluble and can be transported in blood. Chylomicrons (CM): CM is formed in intestine from dietary TAG, cholesterol ester, phospholipids and apoprotein. Function transport dietary (exogenous) TAG and cholesterol from intestine→ blood→ tissues. In blood, lipoprotein lipase hydrolyses TAG carried on CM leaving what is called chylomicron remnant which deliver dietary cholesterol into liver. So, CM function to deliver dietary TAG to adipose tissue and muscle and dietary cholesterol to the liver. VLDL transport endogenous lipids (synthesized TAG and cholesterol) from liver to blood to tissues. LDL transport mainly cholesterol from blood to tissues or organs that require cholesterol for membrane structure or steroid hormone synthesis. High LDL indicates high plasma cholesterol so it is called: Bad cholesterol HDL transfer excess cholesterol from tissues to liver to be excreted in bile as free cholesterol or converted into bile acids or passed into intestine and excreted with faces. This process is called “reverse cholesterol transport. HDL is called Good cholesterol. Synthesized TAG and Choleserol -Chylomicrons are the lowest of lipoproteins in density and the largest in size, contain the highest lipid and smallest protein contents. Neutral fat (TG) % Protein % Phospholipid % Cholesterol % Type of apoprotein CM 85 VLDL 60 LDL 8 HDL 3 2 8 10 15 22 20 50 30 5 15 50 17 apo E apo C apo E apo C apo B apo A-1 apo E apo C -VLDLs and LDLs are more dense, having higher content of protein and lower content of lipid - HDL is the most dense, contain the lowest amount of lipid and the highest of protein. Plasma lipoproteins and atherosclerosis (accumulation of cholesterol in the inner surfaces of large and medium sized arteries): LDL carry most of the cholesterol in the blood, thus, the more LDL-cholesterol in blood, the greater risk of heart disease. HDL represents the removal of cholesterol from tissues thus: decreased HDL is associated with atherosclerosis and coronary heart disease and increased HDL protects against atherosclerosis and coronary heart disease LDL/HDL ratio help in predicting atherosclerosis and coronary heart disease: ↑ LDL/HDL → atherosclerosis ↓ LDL/HDL → No atherosclerosis and Finally!!