* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thrombophilia
Survey
Document related concepts
Transcript
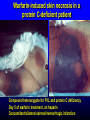
INHERITED THROMBOPHILIA INHERITED THROMBOPHILIA Defects in physiologic anticoagulant pathways •Antithrombin deficiency •Protein C deficiency •Protein S deficiency •Factor V Leiden Increased production of procoagulant •Prothrombin G20210A gene mutation Many other genes affect coagulation – the contribution of mutations in these genes to thrombotic risk is presently impossible to quantify INHERITED CONDITIONS NOT ASSOCIATED WITH CLINICALLY SIGNIFICANT RISK OF THROMBOSIS • MTHFR mutations • Plasminogen deficiency and other defects in fibrinolysis THROMBOPHILIA DUE TO DEFICIENCY OF ANTICOAGULANT PROTEIN Antithrombin, Protein C, Protein S • Typically 30-60% of normal plasma activity of • • affected protein Dominant inheritance Genetically heterogeneous Type 1: low antigen and activity Type 2: normal antigen, low activity (missense mutations) Type 3 (Protein S): Increased binding to C4b binding protein, decreased free protein S level/activity • Thrombotic risk varies from family to family • Together account for approximately 10-15% of cases of inherited thrombophilia Plasma levels of anticoagulant proteins are poor predictors of inherited deficiency Likelihood that a gene mutation is present if measured protein activity is 50% of normal: – Antithrombin = 75% – Protein C =60% – Protein S = 25% Thromb Haemost 2012; 108: 247 FACTOR V LEIDEN A highly prevalent inherited risk factor for thrombosis • Polymorphism eliminates a preferred protein C cleavage site, slows inactivation of factor Va by protein C • Factor Va procoagulant activity not affected Not a “deficiency” • Same genotype responsible for all cases Diagnosed by DNA testing • Very common About 5% of US population heterozygous, 0.05% homozygous High allele frequency implies evolutionary advantage FACTOR V LEIDEN Prevalence in different ethnic groups Group Allele frequency Heterozygote frequency Homozygote frequency European 4.4% 8.6% 0.2% Asia Minor 0.6 1.2 0.004 African 0 0 0 SE Asian 0 0 0 Native American 0 0 0 Lancet 1995;346:1133 FACTOR V LEIDEN INCREASES RISK OF VENOUS, BUT NOT ARTERIAL, THROMBOSIS Physicians' Health Study (15,000 subjects) Ridker et al, NEJM 1995;332:912 P = 0.02 12 % Heterozygotes 10 8 P = 0.9 6 P = 0.7 P = 0.4 4 2 None MI Stroke MI or Stroke 0 Type of thrombosis DVT or PE PROTHROMBIN G20210A GENE POLYMORPHISM • Polymorphism in 3' untranslated (noncoding) part of prothrombin gene • No effect on prothrombin structure or function • Heterozygotes have 5-10% higher plasma levels of prothrombin, 2-3 fold relative risk of venous thromboembolism • About 1-2% of population heterozygous • Diagnosis: DNA testing CLINICAL FEATURES OF INHERITED THROMBOPHILIA INHERITED THROMBOPHILIA Clinical findings in homozygous state Antithrombin III deficiency CLINICAL FINDINGS IN HOMOZYGOTES lethal? Protein C deficiency neonatal purpural fulminans Protein S deficiency neonatal purpural fulminans (? - rare) FVL Prothrombin mutation Premature thrombosis in many (most?) - some asymptomatic CONDITION HOMOZYGOUS PROTEIN C DEFICIENCY WITH NEONATAL PURPURA FULMINANS INHERITED THROMBOPHILIA Clinical Features in Heterozygotes • Venous thromboembolism No convincing evidence of increased risk of arterial thrombosis • Onset often in 20s and 30s Many carriers asymptomatic throughout life • About half of VTE episodes unprovoked • Increased risk of pregnancy loss Thrombotic risk varies with type of thrombophilia Proportion of individuals from thrombphilic families who have an episode of VTE by age 50: • Protein C, protein S or antithrombin deficiency: 40-50% • Factor V Leiden or prothrombin polymorphism: 10-15% Blood 2009;113:5314 Relative risk of VTE in individuals with low plasma protein S vs protein S mutation Relative risk of thrombosis 95% CI Low plasma protein S (general population) 0.7 0.3-1.8 Protein S mutation in thrombophilic family 11.5 4.3-30.6 Koster et al, Blood 1995;85:2756 Simmonds et al, Ann Intern Med 1998;128:8 Family history predicts thrombotic risk just as well as laboratory testing for thrombophilia A case-control study Fam Hx VTE Odds ratio for thrombosis (95% CI) Negative 1 (reference) Any relative 2.2 (1.9-2.6) Relative < 50 2.7 (2.2-3.4) > 1 Relative 3.9 (2.7-5.7) Event OR for event with positive FH OR for event with positive test for thrombophilia Unprovoked VTE 2.5 2.3 Provoked VTE 16.4 21.2 Arch Intern Med 2009;169:610 The presence of thrombophilia does not predict thrombotic risk in the absence of a family hx of VTE Event in proband leading to diagnosis of thrombophilia Incidence of VTE in relatives per 1000 patient-yrs (95% CI) Carriers Non-carriers VTE 1.6 (1.2-2.2) 0.5 (0.3-1.0) Arterial thrombosis 0.5 (0.1-2.8) 0.8 (0.2-3.0) Obstetric complication 0.6 (0.2-1.6) 0.0 (0-0.8) Asymptomatic 0.3 (0.1-1.2) 0.0 (0-0.7) Thromb Haemost 2011;106:646 RISK OF THROMBOSIS IN THROMBOPHILIA EFFECT OF GENE DOSE Relative risk of thrombosis in heterozygous and homozygous factor V Leiden Genotype Relative Risk Normal 1 Heterozygous 7 Homozygous 80 Rosendaal et al, Blood 1995;85:1504 EFFECT OF GENE INTERACTIONS Co-inheritance of protein C deficiency and factor V Leiden within a family Gene Mutation Thrombosis present Thrombosis absent (%) (%) Protein C and Factor V 16 (73) 6 (27) Protein C 5 (31) 11 (69) Factor V 2 (13) 11 (87) None 0 11 (100) Koeleman et al, Blood 1994;84:1031 INTERACTION WITH ACQUIRED RISK FACTORS Oral contraceptive RISK FACTOR RELATIVE RISK OF THROMBOSIS Oral contraceptive 4 Factor V Leiden 8 Both 35 Vandenbroucke et al, Lancet 1994;344:1453 INTERACTION WITH ACQUIRED RISK FACTORS Estrogen replacement RISK FACTOR RELATIVE RISK OF THROMBOSIS Estrogen replacement 3.5 Factor V Leiden 4.6 Both 11 Rosendaal, 2001 WHAT ARE THE IMPLICATIONS OF A POSITIVE TEST FOR THROMBOPHILIA IN AN ASYMPTOMATIC PERSON? RELATIVE RISK OF VENOUS EVENTS IN RELATIVES OF PATIENTS WITH THROMBOPHILIA Vossen et al, J Thromb Haemost 2004;2:1526 THE ABSOLUTE RISK OF VENOUS EVENTS IN ASYMPTOMATIC RELATIVES OF THROMBOPHILIC PATIENTS IS LOW Vossen et al, J Thrombos Haemost 2005;3:459 Bleeding risk with long term anticoagulation estimated at 1-3%/year INCIDENCE OF FIRST VTE EVENTS IN SPECIFIC RISK SITUATIONS IN THROMBOPHILIC INDIVIDUALS Vossen et al, J Thrombos Haemost 2005;3:459 THROMBOPHILIC INDIVIDUALS CONTROLS Travel > 8h 0% (0/504) 0% (0/1244) Surgery or immobilization > 2 w 2% (3/176) 0.04% (2/407) Plaster cast 0% (0/33) 0% (0/71) Cancer 10% (1/10) 6% (1/17) Pregnancy 7% (2/28) 0% (0/75) RISK SITUATION Analysis restricted to individuals not given prophylaxis SHOULD ORAL CONTRACEPTIVES ROUTINELY BE WITHHELD FROM WOMEN WITH FACTOR V LEIDEN? PREDICTED OUTCOMES WITH ALTERNATIVE CONTRACEPTIVE METHODS Oral contraceptive Levonorgesterol -IUD Copper IUD Condom 1st VTE/100 pregancy-yr 0.55 0.25 0.25 0.25 VTE/100,000 pregnancy-yr 550 250 250 250 Unintended pregnancies/ 100,000 p-y 200 700 1400 12,000 Additional cases of VTE 6 20 40 336 Total # VTE 556 270 290 586 Blood 2011;118:2055 MANAGEMENT OF ASYMPTOMATIC INDIVIDUALS WITH INHERITED THROMBOPHILIA • • • Counseling/reassurance Prophylaxis in high-risk situations Carefully consider risk/benefit ratio and alternatives when prescribing oral contraceptives or HRT IS THE MANAGEMENT OF PATIENTS WITH VTE AFFECTED BY THE RESULTS OF THROMBOPHILIA TESTING? The risk of recurrent venous thromboembolism is higher in patients with idiopathic events Lancet 2003; 362: 523–26 Idiopathic VTE Other risk factor Postop VTE The risk of recurrent VTE is not significantly affected by the presence of inherited thrombophilia Lancet 2003; 362: 523–26 Hazard ratio 1.50 (95% CI = 0.82-2.77) p=0.187 The presence of inherited thrombophilia does not usually affect treatment of patients with VTE • • Idiopathic VTE is a strong independent predictor of recurrence risk, and so is a potential indication for long-term anticoagulation The presence of inherited thrombophilia is not a good predictor of VTE recurrence risk and so should not be used as the basis for prolonging therapy Warfarin-induced skin necrosis in a protein C-deficient patient Compound heterozygote for FVL and protein C deficiency Day 5 of warfarin treatment, on heparin Concomitant bilateral adrenal hemorrhagic infarction WARFARIN LOWERS LEVELS OF PROTEIN C FASTER THAN LEVELS OF PROCOAGULANT VITAMIN K-DEPENDENT PROTEINS Prothrombin Protein C Transient hypercoagulability? THROMBOPHILIA AND PREGNANCY INCREASED RISK OF FETAL LOSS IN WOMEN WITH HERITABLE THROMBOPHILIA European Prospective Cohort on Thrombophilia (1384 women) Lancet 1996;348:913 CONDITION RR OF STILLBIRTH 95% CI RR OF MISCARRIAGE 95% CI ANTITHROMBIN DEFICIENCY 5.2 1.5-18.1 1.7 1.0-2.8 PROTEIN C DEFICIENCY 2.3 0.6-8.3 1.4 0.9-2.2 PROTEIN S DEFICIENCY 3.3 1.0-11.3 1.2 0.7-1.9 FACTOR V LEIDEN 2 0.5-7.7 0.9 0.5-1.5 COMBINED DEFECTS 14.3 2.4-86.0 0.8 0.2-3.6 ALL THROMBOPHILIA 3.6 1.4-9.4 1.27 0.94-1.71 BUT… There is no evidence that anticoagulant (LMWH) or antiplatelet (ASA) prophylaxis improves pregnancy outcomes in women with inherited thrombophilia WHO TO TEST? Inherited thrombophilia is more likely if a patient with VTE Is young Has a family history of VTE Had unprovoked VTE Had warfarin-induced skin necrosis (protein C) Test results rarely affect patient management! WHEN TO TEST? • • • • FVL, prothrombin polymorphism: any time (DNA test not informative after liver transplantation) Antithrombin: Not during acute thrombosis Not during pregnancy or estrogen/OCP use Off heparin/LMWH at least 2 weeks Protein C: Off warfarin (preferable), or on stable warfarin dose at least 2 weeks Preferably not during acute thrombosis Protein S: As for protein C Not during pregnancy, OCP use or acute inflammation Neonatal period, DIC, liver disease, asparaginase Rx can all cause acquired deficiency of AT, PC, PS Testing should usually be done in the outpatient setting ACQUIRED THROMBOPHILIA • Antiphospholipid syndrome • Hyperhomocysteinemia (may be inherited) • Inflammatory bowel disease • Cancer • Nephrotic syndrome • Myeloproliferative disorders • Pregnancy • Oral contraceptive/estrogen • Hyperviscosity HOMOCYSTEINE CAUSES OF HYPERHOMOCYSTEINEMIA SEVERE • homozygous cystathione beta-synthase deficiency (1:250,000) • homozygous methylenetetrahydrofolate reductase deficiency MILD OR MODERATE • heterozygous CBS deficiency (0.3-1.4% of population) • C677T polymorphism in MTHFR (10% of population homogyzous) • B12, folate or B6 deficiency • Aging • Chronic renal failure HIGHER HOMOCYSTEINE LEVELS ARE ASSOCIATED WITH VASCULAR RISK Meta-analysis Condition Increase in risk per 5 micromole increase in plasma HC (95% CI) Ischemic heart disease 1.32 (1.19-1.45) Stroke 1.59 (1.29-1.96) VTE 1.60 (1.15-2.22) BMJ 2002;325:1202 BUT… LOWERING HOMOCYSTEINE DOES NOT DECREASE VASCULAR RISK • VISP trial (JAMA 2004): Moderate reduction in HC • • • had no effect on vascular risk during 2 yr followup HOPE 2 trial (NEJM 2006): Vitamin supplements lowered HC levels but had no effect on vascular risk NORVIT trial (NEJM 2006): More aggressive vitamin supplementation associated with increased vascular risk VITRO study (Blood 2007): Lowering HC did not prevent recurrent VTE ANTIPHOSPHOLIPID ANTIBODIES ANTIPHOSPHOLIPID ANTIBODIES • Lupus anticoagulant • Cardiolipin antibodies (IgG, IgM) • Beta-2 glycoprotein I antibodies (IgG, IgM) • Thrombotic risk associated with higher antibody levels, positive tests for more than one type of antibody INCIDENCE OF ANTIPHOSPHOLIPID ANTIBODIES PATIENT GROUP ANTIBODY TYPE APPROX INCIDENCE SLE LAC 30% SLE aCL 40% Blood donors aCL 2% Healthy elderly aCL 52% Love and Santoro, Ann Intern Med 1990 CLINICAL CONDITIONS ASSOCIATED WITH ANTIPHOSPHOLIPID ANTIBODIES The “antiphospholipid syndrome” • Thrombosis (arterial and venous) • Recurrent fetal loss • Hematologic abnormalities: Immune thrombocytopenia Immune hemolytic anemia Arthritis & Rheumatism 2002;46:1019-27 CATASTROPHIC ANTIPHOSPHOLIPID SYNDROME (Asherson, 1992) • 1% or less of APL patients • Generalized vasculopathy (?thrombotic or • • • • inflammatory) Livedo reticularis Multiple organ system involvement Renal failure Hypertension ARDS CNS Rapid progression; sudden death in some patients Treatment: anticoagulation, plasma exchange, immunosuppresion (sirolimus?) “Although a positive APLA test appears to predict an increased risk of recurrence in patients with a first VTE, the strength of this association is uncertain because the available evidence is of very low quality” Blood 2013;122:817-824 ANTIPHOSPHOLIPID SYNDROME CLINICAL CRITERIA 1. One or more documented episodes of arterial, venous, or small vessel thrombosis (other than superficial venous thrombosis) in any tissue or organ – – 2. Thrombosis must be confirmed by objective validated criteria For histopathologic confirmation, thrombosis should be present without significant evidence of inflammation in the vessel wall Pregnancy morbidity a. b. c. One or more unexplained deaths of a morphologically normal fetus at or beyond the 10th week of gestation, with normal fetal morphology documented by ultrasound or by direct examination of the fetus, or One or more premature births of a morphologically normal neonate before the 34th week of gestation because of: (i) eclampsia or severe pre-eclampsia defined according to standard definitions, or (ii) recognized features of placental insufficiency, or Three or more unexplained consecutive spontaneous abortions before the 10th week of gestation, with maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes excluded J Thromb Haemost 2006;4:295 ANTIPHOSPHOLIPID SYNDROME LABORATORY CRITERIA 1. 2. 3. Lupus anticoagulant (LAC) present in plasma, on two or more occasions at least 12 weeks apart, detected according to the guidelines of the International Society on Thrombosis and Haemostasis (Scientific Subcommittee on LACs/phospholipid- dependent antibodies) Anticardiolipin antibody (aCL) of IgG and/or IgM isotype in serum or plasma, present in medium or high titer (i.e., > 40 GPL or MPL, or > the 99th percentile), on two or more occasions at least 12 weeks apart, measured by a standardized ELISA Anti-ß2 glycoprotein-I antibody of IgG and/or IgM isotype in serum or plasma (in titer >the 99th percentile), present on two or more occasions at least 12 weeks apart, measured by a standardized ELISA, according to recommended procedures APL syndrome considered present if at least one of the clinical and one of the laboratory criteria are present J Thromb Haemost 2006;4:295 TREATMENT OF PATIENTS WITH ANTIPHOSPHOLIPID ANTIBODIES Asymptomatic: no treatment History of thrombosis: – Consider prolonged treatment in selected patients • Recurrent or unprovoked thrombosis (arterial or venous) • Persistently high antibody levels • More than 1 APL antibody test positive – Most patients can be treated with standard anticoagulant regimen • Two RCTs have shown inferior outcomes with high intensity warfarin treatment • A few patients exhibit warfarin failure – consider long term LMWH treatment (no data yet on newer oral anticoagulants) ANTIPHOSPHOLIPID ANTIBODIES AND FETAL LOSS • Antiphospholipid antibodies associated with lower live birth rates in unselected “low-risk” pregnancies • Live birth rates in untreated women with APL and at least one fetal loss have ranged from 10-85% in published studies • Aspirin and heparin have been associated with higher live-birth rates in several studies, but most of these did not include a placebo-treated arm Arth Rheum 2004;50:1028 ANTIPHOSPHOLIPID ANTIBODIES AND FETAL LOSS • Testing for APL should be restricted to women with at least three consecutive miscarriages • Other causes of pregnancy loss (especially abnormal karyotypes) should be ruled out • If criteria for obstetric APL syndrome met, treat with aspirin and/or LMWH during pregnancy and postpartum period Arth Rheum 2004;50:1028 ANTICOAGULATION IN WOMEN WITH APLA AND RECURRENT PREGNANCY LOSS 2012 ACCP CONSENSUS RECOMMENDATIONS • Women who meet lab and clinical criteria for obstetric APLA: Antepartum prophylactic or intermediatedose LMWH plus low dose ASA CHEST 2012; 141:e691S