* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Severe Intoxication with the Veterinary Tranquilizer Xylazine in
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Intravenous therapy wikipedia , lookup
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
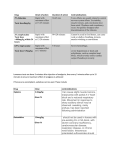
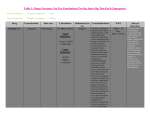
Journalof AnalyticalToxicology,Vol. 25, May/June2001 Severe Intoxication with the Veterinary Tranquilizer Xylazine in Humans U. Hoffmann 1,*, C.M. Meister 2, K. Golle2, and M. Zschiesche 1 1Departrnentof Pharmacology, Ernst-Moritz-Arndt University Greifswald, Friedrich-Loeffler-Str.23 d, D-17489 Greifswald, Germany and 2Departmentof Anaesthesiology, Central Hospital, Wollweber Str. 21, D- 17109 Demmin, Germany Abstract[ Xylazine (Rompun| Proxylaz | is a veterinary tranquilizing agent. A case of self-injection of 1.5 g xylazine by a 27-year-old farmer is reported. He subsequently became comatose, hypotensive, bradycardic, and mildly glycemlc. An intensive supportive therapy including intubation and ventilation was required. The patient made a full recovery over the next 30 h. The largest concentrations measured were 4.6 mg/L in plasma, 446 mg/L in gastric fluid, and 194 mg/L in urine. The calculated plasma half-life was 4.9 h. Kinetic data correlated with clinical symptoms. Qualitative and quantitative analysesof xylazine were done by thin-layer chromatography, gas chromatography-massspectrometry, and high-performance liquid chromatography. These methods allow the detection"of small amounts substance in stomach, plasma, and urine. Liquid-liquid extraction was used for the isolation of drug. The sensitvity is high, and with these methods, a rapid analysis is possible. Xylazine intoxications in humans are rare. We describe the management of acute poisoningand present a review of xyl;~zine toxicity in humans. Introduction Xylazine hydrochloride is the generic name for [2-(2.6dimethylphenylamino)-4-H-5.6-dihydro- 1.3]thiazine hydrochloride, which is extensively used in veterinary practice either alone as a sedative or in combination with other drugs for sedation, analgesia, or general anesthesia. It is marketed under the trade names Rompun (Bayer) and Proxylaz (Prodivet/Atarost) and is available in 2, 5, and 10% solutions or as dry product containing 500 mg per vial. Chemical structure and pharmacological properties are similar to the phenothiazines and the antisympathonic agent clonidin (Figure 1). Xylazine acts by stimulat~ofi of r in the central and peripheral nervous system. CNg-mediated actions include strong sedation and respiratory depression. Plasma levels of norepinephrine, epinephrine, insulin, and non-esterified fatty acids are decreased, and 9Author tOwhom correspondenceshould be addressed: Dr. Ulrich Hoffmann, Depanrnentof Pharmacology,Ernst-Moritz-AmdtUniversily, F.-L~effler-Str.23 d, D- 17487 Greifswald, Germany. E-mail: jaki@rnaihuni-gceil'swald.de. glucose is increased. The therapeutic index is relatively small (1), and two- to threefold overdose can lead to collapse or death of the animal due to circulatory and respiratory depression (2). Xylazine has also been investigated in humans, but was frequently associated with marked hypotension, and therefore its use is restricted to veterinary medicine. In humans, toxicity consists of fainting, bradycardia, hypotension, hyperglycemia, apnoe, and coma (3-9). Effects of xylazine are discussed by Mittleman et al. (9) and Fyffe (10). We present a case of severe xylazine intoxication with suicidal intention and report on some laboratory and pharmacokinetic parameters and the clinical management of poisoning. Case History The patient, a 27-year-old farmer, attempted to commit suicide by self-administration of about 75 mL 2% aqueous solution xylazine (Proxylaz/Atarost) by intramuscular injection as a consequence of a conflict situation in his family. He was found to be comatose with narrow pupils and no response to light and pain stimuli. His initial heart rate was 88 bpm; blood pressure was 180/100 mm Hg. Two hours after ingestion, he was transported to the Intensive Care Unit of the hospital. After endotracheal intubation, the patient received gastric lavage, activated charcoal, and cathartics. He was placed on a ventilator and received intensive therapy including intravenous fluid therapy and urinary, gastric, and central venous catheters. The patient received 20 mg etomidate i.v., 200 mg propofol i.v., 0.25 mg orciprenaline i.v., 10 mg metoclopramide i.v., and 50 mg ranitidine i.v. The heart rate and the blood pressure decreased in H Xylazlne Figure1. Structuresof xylazineand clonidine. Reproduction(photocopying)of editorialcontentof thisjournalis prohibitedwithoutpublisher'spermission. H CI Clonldlne 245 Journal of Analytical Toxicology, Vol. 25, May/June 2001 the course of the two days, to 50 bpm and 100/40mm Hg, respectively, recovering later gradually. There were hypotensive episodes that responded to intravenous fluid infusion. After admission, plasma glucose was slightly increased, 9.8 mmol/L, but subsequent glucose estimations were normal. The patient had increased bronchial secretion and decreased body temperature of 35.6~ The electrocardiogram showed no abnormalities, and no evidence of myocardial damage was observed. His status gradually improved, and he exhibited episodes of spontaneous movements. The patient regained consciousness and was extubated 20 h after admission. He was discharged four days later and referred to an ambulatory psychiatric attendance. Blood, urine, and gastric secretion were taken immediately after admission to the hospital for laboratory investigations. Analytical Methods Materials All chemicals were of analytical reagent grade9 Xylazine hydrochloride reference standard was generously supplied by Prodivet Pharmaceuticals (Eynatten, Belgium). The internal standard, midazolam, was obtained from Hoffmann-La Roche (Basel, Switzerland). Methods Gas chromatography-massspectrometry (GC--MS).Xylazine was initially detected during drug screening in gastric fluid, serum, and urine. The screening was performed on an HP 5890 GC with a 5972 mass selectivedetector. The chro2 0 . O0 T matographic columns (HP 1) were 15-m x 0.25mm i.d. capillary columns with 0.25-1Jm film thickness. The oven temperature was programmed from 100 to 300~ at 20~ The injector temperature was 270~ the transfer line was held at 300~ Helium was used as carrier gas I,' " 'I'' ata flowrate of 2 mL/min. The injection was made i| i in the splitless mode with 1 min hold time, and -3 9 e . O0 2.00 ' 4'.aa 6'.oe ~e~ee *2:ee 8~.e8 the mass range scanned was 50-550 ainu. T I ~ (rain) Xylazine was detected in samples and compared Figure2. Liquid chromatogram of an extract from plasma sample after xylazine intoxication. Xylazine, with reference spectra obtained from commercial 3.21 min; midazolam, 10.95 min. xylazine solution. Quantitation was carried out using xylazine standards in urine and gastric fluid. Table I. Cases of Xylazine Intoxications in Humans Thin-layer chromatography (TLC). Xylazine was also analyzed by thin-layer chromatography Volume/dose Concentration (TLC) using Toxi-Lab| drug detection system Gender* Age Usage + Application* (mL) resp. (mg) (mg/t) References (Analytical Systems, Laguna Hills, CA).The substance was isolatedfrom urine and gastric fluid in M 34 S i.m. 10 1000 Carruthers et al. Toxi-Tubes A by liquid-liquid extraction at pH 9. 1979 (3) After solvent evaporation, the chromatogram was F 20 S oral 4 400 plasma: neg. Gallanosa et al. developed and the spots detected by sequentially urine: pos. 1981 (4) F 39 A i.m. --Lewis et al. dipping in sulfuric acid, water, viewing under UVserum: 0.03 urine: 1.7 1983 (5) light, and dipping in Dragendorffs reagent. The M 36 S i.v. --serum: 0.2 Poklis et al. xylazine spot was identifiedby comparing with the urine: 7.0 1985 (6) spot of the parent substance. F 29 -- i.m. I 40 (0.73 mg/kg) F 37 S i.m. 24 F M 29 19 -S i.v. s.c. -2 2400 (22 mglkg) -200 F 23 H -- -- M 33 H M 27 S i.m. 75 m -- Samanata et al. 1990 (8) liver: 42 mglkg Mittleman et al. kidney: 28 mg/kg 1997 (9) brain: 19 mglkg liver: 0.26 mglkg kidney: 0.15 mglkg brain: 0.16 mglkg 1500 serum: 4.6 mg/L this work (13 mg/kg) urine: 194 mg/L 2000 stomach: 446 mgiL -- * M: Male, F: Female. * A: accidental intoxication; S: suicide attempt; H: homicide. * i.m.: intramuscular; s.c.: subcutaneous; i.v.: intravenous. 246 Spoerke et al. 1986 (7) High-performance liquid chromatography (HPLC).The HPLCfrom Merck-Hitachiconsisted of an L-2000 pump, an AS-2000autosampler, and an L-4500 diode-array detector. The separation was performed on a LiChroCart 125-4, RP select B column at 25~ The eluent was monitored at 220 nm. The mobile phase, consisting of triethylammonium phosphate (0.02M, pH 3)/acetonitrile (77:23), was pumped at a flowrate of 1 mL/min. Analyses. A 1-mg/mL xylazine stock solution was prepared in methanol. Calibration curves were constructed by means of linear regression using the peak-height ratio between xylazine and midazolam as internal standard from spiked serum blanks of the concentration from 25 to 800 ng/mL. The patient samples were evaluatedby the internal standard method using peak-height ratios for calculation. Relative retention time and Journal of Analytical Toxicology, Vol. 25, May/June 2001 5000 4000 ff 8 3000 o o ~9 2000 m >. X 1000 T r 2 4 6 8 10 Time (hours after ingestion) 12 curve. Figure 3. Plot of xylazine plasma concentrations versus time in humans after intoxication. Plasma halflife t,h = 4.9 h after i.m. adminstration of about 1.5 g xylazine. i plasma xylazlne cholesterol 7.03 11.85 UV spectrum were used for qualitative identification. Sample extraction. Plasma samples were collected every 2 h for a period of 12 h. One milliliter of plasma was spiked with 20 pL midazolam (0.1 rag/L), and 1 mL borate buffer (pH 9.5) was added. The sample was vortex mixed and extracted twice with 3 mL ether. After centrifugation, the organic phases were combined, dried under a gentle stream of nitrogen and redissolved in 3 ml hexane/ethanol (10:4, v/v). After the addition of 200 lJL of water, the upper hexane phase was discarded, the rest evaported completely under nitrogen at 80~ and the extract resolved in 140 IJL mobile phase for injection into the LC. When necessary, plasma samples were diluted to yield concentrations within the limits of the calibration In the case of GC-MS analysis, the urine and gastric juice extracts were resolved in 50 IJL methanol, and 1 IJL was injected into the GC. A Results Time (mln) B TIC:M-037-T1,D ooo(x)oo stomach 6.71 IIdocaine 7 , 1 4 xylazlne 7oc<)cr notxxmo f,t x ~ 40ooooo 3 o ~ 2000000 IO~OOO ~o" ' ' ~..6o" " ;~.~ " " ~.6o" " ' ~ . ~ " ' ++.~" /+.,~ " " ~'.~ " " ~'.~d " " / + . ~ " ' t+.,~ ' ' ~.6d ' "/+.~ " Tlme (mln) 9.+.oo~176176 C la~or urine CH~ 205 1r =M + c ,a +- .........i..I, ,1, ![ ~II ~ ~J ~- ,,.l .- d ' ] :!. :1:. II1- .,.,,, ..... !~, [ m/z Figure 4. Total ion chromatogram of xylazine in plasma (A) and gastric fluid (B) and mass spectrum o f xylazine p e a k in urine (C). The HPLC system described was suitable for the analysis of xylazine in serum. The recovery was 75%. The linear relationship calculated from the peak-height ratio and the xylazine concentration in plasma up to I mg/L was R = -0.16 + 0.0069x (r > 0.99). The relative error of the calibration was between -9.5 and 1 9%. The quantitation limit (LOQ) was 25 ng/mL, the detection limit (LOD) was 5 ng/mL using plasma samples larger than 1 mL. Statistical evaluation of accuracy and precision were not performed. Figure 2 shows a typical chromatogram. Xylazine was detected in all samples analyzed. The toxicological results are presented in Table I. The plot of plasma xylazine concentration versus time after i.m. application is shown in Figure 3. The level of drug concentration declined over 12 h. Further plasma samples were not taken. These data were well fitted by a one-compartment model with an exponential decline. The plasma half-life calculated was t]/2=4.9 h. Gastric fluid, plasma, and urine samples were also analyzed by GC-MS (Figure 4). Xylazine was found in all samples, even in the stomach contents. The retention time and mass spectra were matched with a standard and found to be identical. The parent ion of xylazine is registered at m/z 220. Further ingredients of the original xylazine solution as 4-hydroxy benzoic acid methyl- and -propylester were also found in urine. Xylazine metabolites were not identified. The characteristics of xylazine detected by TLC 247 Journal of Analytical Toxicology, Vol. 25, May/June 2001 are shown in Figure 5. The Rf value amount was 0.75. The color results were as follows: stage I was rose-brown; stage 2 was colorless; stage 3 was faint blue; and stage 4 was brown. Discussion Xylazine is extensively used in veterinary practice as a sedative with analgesic and muscle relaxant properties. The dose in animals is 0.5-5.0 mg/kg i.v. or i.m. and produces bradycardia and respiratory depression. This usually begins within a few minutes and lasts up to 4 h (11). Xylazine produces its effects by stimulation of central (z2-receptors and depression of norepinephrine release from peripheral nerve terminals. As evidenced by the paradoxical blood pressure changes, xylazine possess also peripheral action causing an initial blood pressure increase. The predominating central oe-properties produce inhibitory effects by interneural blockade resulting in long-lasting hypotension, bradycardia, and decreased cardiac output. Xylazine actions may also involve cholinergic, serotonergic, dopaminergic, r histaminergic, or opiate mechanisms (12). Xylazine causes profound respiratory depression, and the present patient, as well as earlier ones (3,4,7,8), required assisted ventilation. The hypotension could be managed by fluid infusion with Sterofundin and Ringer-Lactat solution under the control of electrolytes. Further toxicological signs are sedation, coma, bradycardia, and hyperglycemia. Arrhythmias were not seen. The thermoregulation was disturbed, and body temperature was decreased in the initial phase. Treatment was directed to maintaining respiratory function and blood pressure and was symptomatic. Based on the information from the literature, the e dosages known to produce toxic effects in humans vary from 40 mg up to 2000 mg (Table I). Xylazine is metabolized in the liver and is excreted to 70% renal and to 30% biliary in dogs (2). Studies showed that the drug is metabolized to nearly 20 metabolites, with only 8% of the administered dose being eliminated in the urine as unchanged substance (13). The major metabolite is 2,6-dimethylaniline. In spite of the high dose administered, we did not find any metabolites in the urine of the patient. The xylazine metabolites, especially the aniline derivative, are very volatile substances with short retention times. Our solvent delay time after injection is 3 rain. Therefore, the metabolites might elute together with the solvent before the mass spectrometer was turned on. Because the urine sample was collected very early, the concentration of the metabolites was too small. Pharmacokinetic studies in animals found an exponential decline of xylazine concentration after i.m. dosage. The data were described by an open one-compartment model with a transient absorption phase followed by an elimination phase. Half-life of absorption was short (3--6 rain) during the elimination half-life varied between 30 and 60 min depending on the species (14). We measured plasma concentrations as a function of time and found that the half-life calculated is much larger than in animals if the data were fitted by a first order kinetic model. However, other also models correlate with the experimental data, so an explanation is difficult. Nevertheless, the pharmacokinetic result obtained in the present case is in good agreement with clinical symptoms. Although the substance was self-administered by i.m. injection, a substantial amount was found in gastric fluid, supporting the fact that alkaloids are excreted in the stomach, and a gastric lavage is therefore important. r e A B k 1 2 3 Stage 1 4 1 2 3 Stage 2 4 1 2 3 Stage 3 4 1 2 3 Stage 4 4 Figure 5. Thin-layer chromatogram of xylazine was dipped into four reagents sequentially. Lane 1, substances for comparison; lane 2, urine; lane 3, gastric fluid; and lane 4, 100 lag xylazine. A, lidocaine and B, xylazine. 248 Journal of AnalyticalToxicology,Vol. 25, May/June2001 Various antagonists have been used as a therapy. Tolazoline may be administered in unresponsive bradycardia and hypotension in doses of 10 mg i.v. over an hour up to a maximum of 40 mg (7). On the other hand, tolazoline has been associated with hypertension, arrhythmias, and tachycardia and should be reserved for cases unresponsive to other interventions. Yohimbine, an other c~2-antagonist, has been shown to antagonize the sedative effects of xylazine in humans in doses up to 0.125 mg/kg. It can be used as i.m. injection, which is effective after 15-20 rain, or i.v. injection, after which the effects begin in 1-2 rain (15). Atropine has been used and may reverse bradycardia and hypotension in humans (4). Naloxone was adminstered without effect (7,8). Conclusions Xylazine is a hazardous drug in humans. The plasma concentration in our case is consistent with a fatal overdose and causes coma, respiratory depression, and hypotension. Compared with other human poisonings the clinical findings were similiar and because of the severity of intoxication more pronounced. Intensive supportive therapy is usually necessary. For the detection of xylazine in body fluids both a GC-MS system and an LC method are suitable. TLC is also a convenient and quick screening method for the detection of xylazine. This finding confirmed the results of GC-MS in screening for the drug. The substance can be isolated by liquid-liquid extraction from samples with satisfying cleanliness. The drug is excreted largely by the kidneys. We recommend the need for an awareness of the pharmacological effects of xylazine in humans, especially because of its widespread use in veterinary medicine. Acknowledgment We are grateful to Anja Moll and Gitta Schumacher for skillful technical assistance in the analytical procedures. References 1. Hagers Handbook, part 9, 5th ed., F. von Bruchhausen, G. Dannhardt, S. Ebel, A.W. Frahm, E. Hackenthal, and U. Holzgrebe, Eds. Springer Verlag Berlin, Germany, 1993, pp 1215-1216. 2. E. Rector, K. Otto, M. Kietzmann, I. Nolte and W. Lehmacher. Pharmacokinetics and effects of xylazine (Rompun| in dogs. BerL M~nch. ?ier~rztl. Wschr. 109:18-22 (1996). 3. S.G. Carruthers, M. Nelson, H.R. Wexler, and C.R. Stiller. Xylazine hydrochloridine (Rompun| overdose in man. Clin. Toxicol. 15: 281-285 (1979). 4. A.G. Gallanosa, D.A. Spyker, J.R. Shipe, and D.L. Morris. Human xylazine overdose: a comparative review with clonidine, phenothiazines, and tricyclic antidepressants. Clin. ToxicoL 18:663-678 (1981). 5. S. Lewis and C.L.P.O'Calaghan. Clinical curio: self medication with xylazine. Br. Meal. J. 287:1369 (1983) 6. A. Poklis, M.A. Mackell, and M.E.S. Case. Xylazine in human tissues and fluids in a case of fatal drug abuse. J. AnaL ToxicoL 9:234-236 (1985). 7. D.G. Spoerke, A.H. Hall, M.J. Grimes, B.N. Honea, and B.H. Rumack. Human overdose with veterinary tranquilizer xylazine. Am. ]. Emerg. Med. 4:222-224 (1986). 8. A. Samanta, C. Roffe, and K.L. Woods. Accidental self administration of xylazine in a veterinary nurse. Postgrad. Med. J. 66:244-245 (1990). 9. R.E. Mittleman, W.L. Hearn, and G.W. Hime. Xylazine toxicity-literature review and report of two cases.J. Forensic $cL 43:400-402 (1998). 10. J.J. Fyffe. Effects of xylazine on humans: a review. Aust. Vet. J. 71: 294-295 (1994). 11. S.A. Greene and J.C. Thurmon. Xylazine--a review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 11: 295-313 (1988) 12. J.V. Kitzman, N.H. Booth, and R.C. Hatch. Antagonism of xylazine sedation by 4-aminopyridine and yohimbine in cattle. Am. J. Vet. Res. 43:2165-2169 (1982). 13. A.E. Mutlib, Y.C. Chui, L.M. Young, and F.S.Abbott. Characterisation of metabolites of xylazine produced in vivo and in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos. 20:840-848 (1992). 14. R. Garcia-Villar, RL. Toutain, M. Alvinerie, and Y. Ruckebusch. The pharmacokinetics of xylazine: an interspecific study. J. Vet. PharmacoL Ther. 4:87-92 (1981). 15. C. Macintosh. Potential antidote for Rompun| (xylazine) in humans. NZMed.J. 98:714-715 (1985). Manuscript received July 25, 2000; revision received October 27, 2000. 249