* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hepatitis C and injecting drug use an overview.
Survey
Document related concepts
Transcript
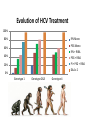
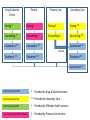
Hepatitis C and injecting drug use an overview. Vivian Hope, Centre for Infectious Disease Surveillance and Control, Public Health England. National Intelligence Network meeting on the health harms associated with drug use 04/06/14. Reported laboratory diagnoses of hepatitis C infection: England Total number of reports: All exposures and exposure not known. Proportion of the reports with exposure data, in which injecting drug use was indicated# 100% Number of reports 10,000 80% 8,000 60% 6,000 40% 4,000 20% 2,000 0 0% 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 Proportion of reports with risk information associated with IDU# 12,000 # Data on exposure is often incomplete or missing. HCV in UK report, 2013; Shooting Up, 2013. Hepatitis C among people who inject psychoactive drugs Rate of sharing Anti-HCV prevalence 100% 80% 60% 40% 20% 0% 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 UAM Survey of PWID Data Tables, 2013. Risk & Incidence The number of adults that had ever injected receiving drug treatment has increased by a third (from 84,216, 2005/06 to 111,939, 2011/12). The vast majority of PWID asked report recent use of a needle & syringe programme (NSP) (83% in 2012, UAM Survey). NSP coverage needs to be improved in England as in 2012: • Only about half of PWID report that the number of needles they had received was greater than the number of times that they had injected (47% during preceding four weeks, UAM Survey). • One-third of PWID report injecting with a previously used needle that they had attempted to clean (33% during last 28 days, UAM Survey). Preliminary data from UAM Survey from 2011-12 indicated that of those who had potentially been at risk of acquiring hepatitis C, 3.3% (95% CI, 2.6%-4.1%) had been infected. Suggesting an incidence of between 7 and 20 infections per 100 person years of exposure in England, Wales and Northern Ireland. HCV in UK report, 2013. Hepatitis C among people who inject image & performance enhancing drugs Prevalence of hepatitis C is lower those injecting among psychoactive drugs Around one in 20 people who image & performance enhancing have ever been infected drugs with hepatitis C. 1.5% are living with HIV 5.5% have been infected with hepatitis C 8.8% have been infected with hepatitis B Only 9% of those who inject image and performance enhancing drugs reported ever sharing injecting equipment. Shooting Up, 2013. Uptake of voluntary confidential testing for hepatitis C: England Proportion aware of their hepatitis C infection People who inject psychoactive drugs Voluntary confidential testing uptake Proprtion of those tested, tested during preceding two years 100% 80% 60% 40% 20% 0% 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 Only 22% of those who inject image and performance enhancing drugs report uptake of voluntary confidential testing for hepatitis C. HCV in UK report 2013, Shooting Up 2013. Acknowledgments: Fortune Ncube, Katelyn Cullen, Helen Harris & Sema Mandal at the Centre for Infectious Disease Surveillance and Control. Thank you. Opt-out BBVs testing policy in prisonspublic health opportunities & challenges. Dr. Autilia Newton Deputy Director for Health & Justice, PHE Prevalence of disease: prison/community 2 PWID, HCV and Prisons •Injecting drug use continues to be the most important risk factor for HCV infection in the UK. •Data from the Unlinked Anonymous Monitoring (UAM) survey of people who inject drugs (PWID) suggest that levels of infection in this group remain high in 2012 (49% in England, 34% in Northern Ireland and 33% in Wales); •Two-thirds (69 %) of prisoners have used at least one drug during the year •About one third of all people treated for substance misuse in England are treated in prisons (60,000 prison clinical drug treatment episodes p.a./197,110 community treatment contacts 2011-12); •So prisons are a good setting to test and treat people for BBVs, especially HCV. •Trend in HCV prevalence* among PWID in England & Wales: 2002-2012 ‘Community Dividend’ for public health interventions in prison populations • Underserved populations passing through prison estate ~160,000 per year; • Often belong to wider social groups and networks contributing significantly to health inequalities generally; • Delivering health interventions in prisons not only benefits prisoners’community dividend’ in addressing issues in underserved populations generally. BBV testing data in prisons Prison Health Performance Quality Indicators (PHPQIs) Collated quarterly by NHS Trust Development Authority Collects data on number tested and percentage of hep C tests performed out of total number of receptions in that year • PHE Sentinel Surveillance of BBV testing Reports on trends in BBV testing across England in the 24 participating laboratories • Genitourinary Medicine Clinic Activity Dataset (GUMCAD) Captures all STI diagnoses & sexual health service use in GUM clinics “Z” code introduced in 2011 to capture offender data • PHiPs reports PHiPs Team receive reports on positive cases of hep B and C in prisons 5 What we know ……. Trends in individuals tested and testing positive for HBsAg, anti-HCV and HIV 2008-2012 Source: PHE Sentinel Surveillance of BBV testing (across England in the 24 participating laboratories N: submitting = Apx 39 prisons) 2008 2010 2011 2012 Total No. tested No. +ve No. tested No. +ve No. tested No. +ve No. tested No. +ve No. tested No. +ve No. tested No. +ve HBsAg 2,415 38 (1.5%) 2,992 42 (1.4%) 2,860 31(1%) 3,463 52 (1.5%) 3,441 60 (1.7%) 15,171 223 (1.4%) AntiHCV 2,902 592 (20%) 3,384 563 (16.6%) 3,171 469 (14.7%) 4,432 523 (11.8%) 4,267 456 (10.6%) 18,156 2,603 (14%) 631 8 915 10 1,156 7 1,946 11 2,131 14 (0.6%) 6,779 50 (0.7%) HIV 6 2009 PHiPs Team reports (+ve hep B) 7 2010 2011 2012 2013 Hepatitis B acute 2 2 1 0 Hepatitis B chronic 22 45 93 94 PHiPs Team reports* (+ve hep C) * The increase in reports is partly due to the improved reporting system of infectious diseases by HPTs Hepatitis C acute Hepatitis C Hepatitis C (PCR – confirmation of infection) 8 2010 2011 2012 2013 0 1 0 2 106 289 417 735 9 89 205 670 SOPHID During 2011: 9 • 196 patients who attended HIV care were resident in a prison at that time • 481 patients who attended HIV care in 2011 were prisoners at any given time • From 2006-2011, 735 people were resident in prison at the time they attended HIV care services PHPQI hepatitis C testing data 10 PHPQI data 2012-13 Total Receptions Hep C Tests % Hep C tests performed England total 196,374 12,322 6.3% Receptions to English prisons in 2012 who received a hepatitis C test Hepatitis C test results are known to be under-reported Public Health England (2013). Hepatitis C in the UK: 2013 report. http://www.hpa.org.uk/Publications/InfectiousDiseases/BloodBorneInfections/HepatitisCInTheUK/1307HepatitisCintheUK2013report/ 11 Hepatitis C in the UK 12 Care pathways are being developed but we need to do more… In the 2011 survey of hepatitis C services in English prisons,* 82/110 responding prisons (74%) had a written pathway in place to describe what happens following a positive hepatitis C result. National audit** suggests that the most common model of service delivery in English prisons is hospital outpatient care (52% of prisons), followed by hospital in-reach (43%) and GP led care (5%) *Department of Health, Health Protection Agency. National survey of hepatitis C services in prisons in England, 2012. (2012). Available at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/PrisonInfectionPreventionTeam/Guidelines/ **Humphrey C, Professor Lombard M, Dr Newton A, Dr O'Moore E, Railton C. (2013). Public Health England, Department of Health. An audit of Hepatitis C services in a representative sample of English prisons, 2013. Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317139084753 Hepatitis C in the UK 13 Tripartite Agreement: NOMS, NHS England & PHE • Published October 2013: www.justice.gov.uk/about/noms/working-withpartners/health-and-justice/partnership-agreement • • Sets out the shared strategic intent and joint corporate commitments in the commissioning, enabling and delivery of healthcare services in adult prisons in England. The agreement follows the reforms to the Health System in England from April 2013 and replaces the previous National Partnership Agreement between the Department of Health and HM Prison Service (2007). It will remain a live document updated as required but substantively reviewed once a year and will be overseen by a shared Prison Healthcare Board (England). TB Oversight Group Meeting, January 2014 • The agreement sets out: • respective roles and objectives of each organisation in commissioning, enabling and delivering prison healthcare services (including substance misuse services) • shared principles and objectives • shared development objectives • joint governance arrangements • Agreed approaches, shared outcomes and joint principles; • Identified 12 specific developmental priorities on which to work in 2013/14: 14 Tripartite Agreement: Joint Developmental Priorities for 2013-14 • Developing core service specifications for prison health and wellbeing services. • Developing Information Sharing Agreements and processes to drive transparency and continuous improvement of services. • Reducing smoking amongst prisoners and supporting the development of smoke free prisons. • Reviewing the prescribing and abuse of prescription medications. • Improving continuity of care across transitions; • Reviewing multi-agency approaches to managing serious risk of harm. • Reviewing the current commissioning arrangements for healthcare services in private finance initiative (PFI) prisons. • Reviewing the current arrangements for the provision of integrated health and social care services for prisoners. • Reviewing & clarifying future responsibility for the funding of specific healthcare assets and enabling services. • Improving the detection and management of tuberculosis among prisoners at or near reception. • Testing ‘through the gate’ substance misuse services as part of the Transforming Rehabilitation Strategy. • Implementing an ‘opt out’ policy for testing for blood-borne viruses (BBVs) and developing care pathways for those found to be infected. Our objective: • To oversee the implementation of the joint development priority number 12 of the tripartite agreement: NHS England, NOMS and PHE will work together to design and deliver an appropriate ‘opt out’ model of testing for BBVs by April 2014 How? To design a template pathway with accompanying supporting documents for the prison estate, and provide guidance to stakeholders, including commissioners and service providers. 15 BBV Opt-out Testing policy Moving from “at risk selective policy” Universal offer of testing 16 BBVs in Prisons- Primary Care Conference Birmingham May 22 2014 Opt-out BBVs (Hepatitis B, hepatitis C and HIV) testing policy • BBVs testing to be recommended to all prisoners • Prisoners to “opt-out” (i.e. refuse one/more/all tests) if do not wish to be tested • Policy should increase considerably (example of antenatal HIV & Hep B testing) both offer and up-take of testing (current Hep C testing up-take in prison at around 6%) • Policy should result in rationalisation of BBVs testing in prisons, avoiding repeated, unnecessary testing • “Getting it right” in the remand prisons, should leave little testing to do in the training prisons 17 BBVs in Prisons- Primary Care Conference Birmingham May 22 2014 Opt-out BBVs (Hepatitis B, C and HIV) Testing Algorithm All new arrivals (within first month of arrival)* Hepatitis B *BBV testing should be recommended to all prisoners, including those already in prison Vaccination status Recorded: complete course Recorded: incomplete course Vaccination status unknown HBsAG (one off test) + 1 dose vaccine HBsAG (one off test) + 1 dose vaccine (if incomplete course) Negative (complete vaccination course if necessary) Unvaccinated Positive Positive Negative Suspend vaccination & refer for further testing to specialist service Immune Reassure + harm minimisation. Complete course if incomplete vaccination. No need to repeat test. Remember 4th dose at 1 year, ideally a booster at 5 years Complete vaccination course © Crown copyright Hepatitis C HIV BBV testing should be recommended to all prisoners, including those already in prison HCV Ab test BBV testing should be recommended to all prisoners, including those already in prison HIV (Ag +Ab P24) test [screening test- shows exposure] NEGATIVE POSITIVE NEGATIVE POSITIVE (both/either test) Confirmed by confirmation test Reassure+ Harm minimisation Refer to specialist service PCR/Ag test (same sample) [confirmation testshows infection] NEGATIVE POSITIVE Reassure + harm minimisation Reassure + harm minimisation Acute\chronic infection Repeat risk assessment (and recommend test) after each risk event or every 12 months Repeat risk assessment (and recommend test) After each risk event or every 12 months Refer to specialist service Repeat risk assessment (and recommend test) After each risk event or every 12 months © Crown copyright Pathfinder prisons • We know that there are currently exemplars of good practice in the prison estate; • Innovative programmes including use of DBST, in-reach treatment programmes, opt-out testing protocols etc.; • Need to ‘learn from doing’- identification of ‘pathfinder prisons’ by NHS England, NOMS & PHE during 2014-15 on rolling implementation; • From experiences there, will inform roll-out across entire prison estate. 20 Pathfinders so far Y&H – Leeds & Hull North West (Manchester, Kirkham, Forest Bank , Buckley Hall) East Midlands (Nottingham, Ranby, Leicester, Stocken) South West (Dartmoor, Bristol, Channing Wood, Eastwood Park, Leyhill, Earlstoke) 21 Access to treatment for HCV Factors influencing access to treatment • Access to specialist service & type of service (in-reach/out-reach) • Waiting time (currently 18 weeks but new service specification proposal of 6 weeks) • Suitability of patient for treatment • Willingness of patient to undertake treatment • Side effects • New drugs 22 BBVs in Prisons- Primary Care Conference Birmingham May 22 2014 Treatment demand Impact assessment being looked at currently – AT level ….. • What will be the demand, hep C as an example: o Of 100 people tested apx. 70 will have used drugs = 70 people (Ref: Stewart et al 2008, 68% had used an illicit drug in the past year and 40% had injected a drug during the four-week period prior to custody) o Of the 70 apx. 50% known to be +ve = 35 people o Of the 35 apx. ¼ clear naturally = 27 people o Of the 27 apx. half be referred for specialist asst = 14 people o Of the 14 apx. ½ will be assessed = 7 people o Of the 7 apx. ½ will commenced treatment = 4 people o Approximately 4% of people entering prison will go on to access treatment 23 Monitoring the implementation: H-JIPs • Pathfinder prisons: Recommend testing for HBV/HCV/HIV to ALL eligible prisoners within 4 weeks of reception • HCV PCR test to be requested on ALL HCV ab +ve tests • All prisoners found to be chronically infected with Hepatitis B and/or Hepatitis C to be referred to a specialist service and seen within 18 weeks of referral • All prisoners found to be HIV +ve to be referred to a specialist service and seen within 2 weeks of referral 24 Things to consider: 25 • Many people diagnosed in prison will not commence treatment in prisons; • Modality of testing : venous blood; DBST; saliva testing (NOT recommended) • Some prisoners might start treatment in prisons but finish it in the community; • Training of prison staff-health care & wing staff: • Impact of ‘bench-marking’. • Link with services in the community • essential to construct appropriate care pathway; • Complexity of commissioning; • Specialist service model provision (in-reach vs referral out) • Promotion materials (leaflets/posters): no specific fund available for new material, but prisons already accessing material currently available; Data quality- SystmOne, H-JIPs, PHE data sources. Health & Justice Pathway England (April 2013) Police Custody & Courts (Including Liaison and Diversion) Prison (Remand and Sentenced) Adult Offender (Male / Female) Community Order / Release on License Pre or post contact with CJS (General Population) NHS England (Primary Care – GP Services) Young Adult Offender (Male / Female) NHS England (Health and Justice Commissioner) Clinical Commissioning Group (Secondary Care incl. Mental Health services) Substance Misusing Offender (Male/ Female) Local Authority (Public Health including Substance Misuse Services and Social Care) Commissioning Opportunities. • Reducing health inequalities across most vulnerable patient groups • Contributing to the reduction of premature deaths • Cost savings of BBV care further down the care pathway through early identification and intervention • Patient safety management and reducing risk of ongoing transmission • Building on improved pathways that exist in some areas/prisons 27 Commissioning Challenges. • Impact analysis • Shaping the money around demand not demand around the money • Scoping and developing with a constrained budget • What do we stop doing? • Staff skills development • Workforce availability both within prisons and secondary care centres: In reach vs outreach • Continuity of care 28 Conclusions • Tackling BBVs: share partnership public health priority; • Prison ideal setting to tackle high prevalence & inequalities; to enable “ripple” effect in the community at large • Opt-out testing policy: milestone in the fight against BBVs • Challenges: complexity of intervention; complexity of mechanisms to enable implementation; complexity of links with services in the community. 29 Increased uptake and new therapies urgently needed Modelling the predicted impact of treatment under different scenarios Ross Harris Dr. Helen Harris Immunisation, Hepatitis and Blood Safety Department CIDSC, PHE, London Modelling the predicted impact of treatment 1 The burden of HCV • Prevalence of chronic infection in England: 160,000 adults in 2005 • HCV-related end-stage liver disease and deaths have increased steadily over the last 15 years (PHE 2013) –Will disease burden continue to rise? –Can this be averted? Modelling the predicted impact of treatment End-stage liver disease deaths, 1996-2012 Figure reproduced from Hepatitis C in the UK: 2013 report. Public Health England (2013) 2 Back-calculation • Basic idea: – Estimate past incidence and numbers progressing through a natural history model that would give rise to the observed endpoint data • Ingredients: – Observed end-point data over time HES data on ESLD, HCC; ONS HCC mortality – Progression probabilities Reported estimates from cohort studies • Result: – Predicted history of disease-stage structure – Future predictions of disease burden Modelling the predicted impact of treatment 3 Natural history model δ1 Infection Mild Chronic HCV δ2 Moderate Chronic HCV δ3 Cirrhosis 1-δ1-γ1 γ1 Recovery Mortality Non-liver δ5 δ4 Decomp. Cirrhosis γ2 Mortality liver related δ6 HCC γ3 HCC Mortality (Liver transplant states omitted for clarity) PREDICTIONS: Currently infected population progresses toward disease endpoints unless treated • Successful treatment (SVR) assumed to halt progression • Treatment failures not retreated • Future incidence: approx 5,000 cases per year Modelling the predicted impact of treatment 4 Key features of PHE model • Statistical model Back calculation model in a Bayesian framework Progression probabilities as informative priors • Fitted to age-specific ONS/HES data • Assumptions of past incidence not required (although estimate of current prevalence is used as a constraint) Evidence consistency – Fits with the overall picture of observed data, current prevalence and best estimates of disease progression in UK-based cohort studies Predictions under different treatment scenarios • Levels of treatment –Maintaining current levels (3% treated annually*) –100% increase over next 10 years –Scale up to complete coverage over next 10-15 years • Types of treatment –Standard treatment (peg interferon and ribavirin) 37% SVR for genotype 1 and 70% non-1 Worse for older patients/more advanced disease –Improved treatments over next 5 years 90% SVR rate, 60% in cirrhotics *sales/dispensing data 2006-2011; Hepatitis C in the UK 2013, PHE Modelling the predicted impact of treatment 6 Standard treatment Improved treatment 2500 2000 1500 1000 500 0 2015 2020 2025 2030 2035 2040 2015 2020 2025 2030 2035 2040 Year Current levels 100% increase Rapid complete coverage Previous treatment only Modelling the predicted impact of treatment 7 Disease burden and treatment • Disease burden is likely to rise in short term • Increasing treatment levels will mitigate this, but short-term rises seem inevitable • Improved treatment will help to make more immediate impact – Currently those at highest risk of severe disease have low probability of achieving SVR • Swift action required as the infected population approaches advanced disease stage – Modelling indicates greater impact for treating sooner rather than later Modelling the predicted impact of treatment 8 Costs • Existing NICE approved therapies are cost effective EXAMPLE OF COSTS TO SCALE UP DUAL THERAPY Total healthcare costs of infected population £4680 million over next 30 years 3% treated per year Additional cost under current costs £1460 million over next 30 years Not as much as you might think..? Complete coverage of treatment over next 10-15 years • New drugs generate healthcare savings and have greater benefits but will cost more (cost effectiveness yet to be assessed by NICE) Modelling the predicted impact of treatment 9 Implementation • Diagnostic and treatment services need to be accessible to those who need them • If NICE recommendation of new drugs takes place, they are still expensive – Affordability vs. cost-effectiveness – Advocacy and local commissioning needs to be strong, with treatment uptake and outcome data collected for local and national monitoring • Oral treatment regimens with good safety/tolerability profiles that can be rolled-out in community settings will be key Modelling the predicted impact of treatment 10 Is elimination feasible? • High levels of treatment will reduce HCV prevalence as well as disease burden • Reduction in incidence likely – Not modelled in this study, but has been demonstrated1 • What is the level of continued risk from injecting drug use? – Risk of re-infection? – To what extent is there a core group driving transmission?2 • Any core groups of transmitters would need to be reached by treatment1 – Support to help behaviour change will be key 1. 2. Martin et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? Journal of Hepatology 2011 Magiorkinis et al. Integrating Phylodynamics and Epidemiology to Estimate Transmission Diversity in Viral Epidemics. PLOS Computational Biology 2013 Modelling the predicted impact of treatment 11 Motivation Specific government targets to increase healthy life expectancy and reduce premature deaths (The NHS & PH Outcomes Frameworks, Department of Health 2012/2013) Focus on liver mortality and preventable communicable disease Improve health quality as well as length of life Addresses inequalities issues (Healthy Lives, Healthy People, Department of Health 2010) Marginalised groups, current/former injectors, minority ethnic populations Building a healthy future Long term goals, but attainable and cost-effective Is it really that hard to think 20 years ahead? Modelling the predicted impact of treatment 12 Finally, some key questions to ask locally… Q Has the PHE commissioning template been used to estimate HCV prevalence and numbers eligible for treatment in your local area? Q Is local provision in place to promote and offer testing to people at risk of hepatitis C infection, as recommended by NICE? Q Are care pathways in place to ensure that diagnosed individuals can access NICE recommended treatments and care, particularly for those BME groups at increased risk of infection and those diagnosed in drug services and prisons? Q Do the needle and syringe programmes in your local area provide an appropriate range of services with sufficient coverage, as recommended by NICE? Modelling the predicted impact of treatment 13 Acknowledgements • Michael Sweeting & Daniela De Angelis MRC Biostatistics Unit – Original study concept • Annastella Costella, Sema Mandal, Mary Ramsay Immunisation, Hepatitis and Blood Safety department, Public Health England – Updated analysis/study guidance • ONS/HES data providers Modelling the predicted impact of treatment 14 Additional slides Modelling the predicted impact of treatment 15 Available data for England • Hospital Episode Statistics (HES) 1995-2009 – Episodes of end stage liver disease (ESLD) • varices, ascites or hepatic encephalopathy – Episodes of hepatocellular carcinoma (HCC) • HCC mortality, Office for National Statistics (ONS) 1996-2009 – primary liver cancer or HCC (155.0 or C22.0) plus hepatitis C infection • Prevalence in 2005 – Evidence synthesis estimate of chronic prevalence Modelling the predicted impact of treatment 16 Progression probabilities Acute to chronic infection 0.738 (0.698, 0.773) Various (pooled estimate) Age 0–29 0.017 (0.010–0.028) Sweeting 2006 Age 30–39 0.010 (0.005–0.025) Age 40–49 0.016 (0.007–0.035) Age 50+ 0.054 (0.036–0.080) Chronic HCV to moderate chronic HCV Moderate chronic HCV to cirrhosis Age 0–29 0.008 (0.003–0.026) Age 30–39 0.005 (0.001–0.020) Age 40–49 0.008 (0.002–0.029) Age 50+ 0.029 (0.010–0.079) Cirrhosis to HCC 0.035 (0.024–0.046) Hutchinson 2005 Cirrhosis to decompensated cirrhosis 0.065 (0.040–0.092) Hutchinson 2005 Decompensated cirrhosis to HCC 0.068 (0.041–0.099) Planas 2004 Decompensated cirrhosis to liverrelated mortality (not HCC) 0.186 (0.137–0.250) Hutchinson 2005 HCC to HCC mortality 0.605 (0.545–0.676) Hutchinson 2005 Modelling the predicted impact of treatment Sweeting 2006 17 Limitations/assumptions • Progression probabilities – Comparable with other studies on average, but different age patterns could change results – Age-specific, but no data on progression in those infected longterm (>20 years) • Treatment scenarios do not account for retreatment if no SVR; or re-infection • Uncertainty of long-term outcomes in cirrhotics • Non-HCV mortality in people who inject drugs • Changes in incidence if prevalence is reduced – Explicit modelling of transmission process • Alcohol use, heterogeneity, time-dependence… Modelling the predicted impact of treatment 18 Treatment of HCV in People Who Use Drugs Mark Thursz Male Mortality 1970 - 2005 Main Causes of Liver Disease •Alcohol • Obesity • HCV • HBV • Other (PBC, PSC, AIH, DILI) Rates of HCV Infection in different risk groups 60% 50% 40% 30% 20% 10% 0% PWID Prison inmates (Female) Prison inmates (male) Immigrants (Asia) Immigrants ((Africa) Immigrants (E Europe) Age-Specific Prevalence of HCV Bruggman et al JVH 2014 Chronic HCV: mortality from both hepatic and extrahepatic diseases All causes (n=2,394) 35 Cumulative mortality (%) Liver cancer 30 (n=115) 12 30.1%* Extrahepatic diseases 10.4%* 10 (n=2,199) 20 18 16 25 14 8 12.2% 11.0% 12 20 6 15 12.8% 12.4% 10 8 4 6 10 5 2 0 0 0 2 4 6 8 10 12 14 16 18 20 Follow-up (Years) 4 1.6% 0.3% 0 Anti-HCV+, HCV RNA detectable Lee MH, et al. J Infect Dis 2012;206:469–77. 19.8%† 2 4 6 8 10 12 14 16 18 20 Follow-up (Years) 2 0 0 2 Anti-HCV+, HCV RNA undetectable 4 6 8 10 12 14 16 18 20 Follow-up (Years) Anti-HCV- *P<0.001 for comparison among all 3 groups and P<0.001 for HCV RNA detectable vs. undetectable. †P<0.001 for comparison among all 3 groups and P=0.002 for HCV RNA detectable vs. undetectable. Incidence of HCV vs Future Prevalence of Cirrhosis HCV incidence has peaked but long-term consequences continue to increase, as most people were infected 20–30 years ago Incidence of HCV, by year in the USA, 1982–20091 Historical and projected % incidence of cirrhosis among HCV patients in the USA2 1. CDC. Disease Burden from Viral Hepatitis A, B, and C in the United States. Available at: http://www.cdc.gov/hepatitis/pdfs/disease_burden.pdf. Accessed August 2013; 2. Davis GL et al. Gastroenterology 2010;138:513–21. HCV - complications in Europe until 2030 Germany France Spain England Razavi, Waked, Sarrazin et al., J Viral Hepatitis 2014 in press Increasing Demand for Liver Transplants in Europe Number of liver transplants HCV-related cirrhosis is the commonest indication for liver transplantation in Europe Virus-related disease = single largest indication for liver transplant in Europe 63% HCV-related Year European Association for the Study of the Liver (EASL). The burden of liver disease in Europe. Available at: http://www.easl.eu/assets/application/files/54ae845caec619f_file.pdf. Accessed August 2013. Growth limited by donor availability Evolution of HCV Treatment 100% 80% IFN Mono PEG Mono 60% IFN + RIBA 40% PEG + RIBA 20% PI + PEG + RIBA DAA x 2 0% Genotype 1 Genotype 2&3 Genotype 4 Side Effects of Peg-IFN Ribavirin • • • • • • • • • Fatigue/lethargy Pain Depression Suicide Neutropaenia Thrombocytopaenia Diarrhoea Cough Thyroid dysfunction • Anaemia • Teratogenicity Telaprevir Safety in Cirrhotics (CUPIC-Wk 16) Patients, n (% patients with at least one event) Telaprevir n=292 Serious adverse events (SAEs)* 132 (45.2%) Premature discontinuation Due to SAEs 66 (22.6%) 43 (14.7%) Death Septicemia, Septic shock, Pneumopathy, Endocarditis, Oesophageal varices Bleeding, 5 (2.6%) Infection (Grade 3/4) 19 (6.5%) Hepatic decompensation (Grade 3/4) 6 (2.0%) Asthenia (Grade 3/4) 16 (5.5%) Rash (Grade 3/SCAR) 14 (4.8%) Renal failure 5 (1.7%) (Hézode et al., AASLD 2012) Restrictions for use of Peg-IFN/RIBA/PI • Ineligible – Cirrhosis (CP-B) – Depression – Autoimmune disease • Intolerant Side Effects with DAA Regimens Liver-related mortality or liver transplantation (%) SVR is associated with a reduction in liver-related mortality and HCC Liver-related mortality or liver transplantation P<0.001 N=530 Without SVR With SVR Hepatocellular carcinoma (%) Time (y) Hepatocellular carcinoma P<0.001 N=530 van der Meer AJ, et al. JAMA 2012;308:2584–93. Without SVR With SVR Time (y) SVR is associated with a reduction in all-cause mortality International, multicentre, long-term follow-up between 1990–2003 All-cause mortality (%) 5 tertiary care hospitals in 530 advanced fibrosis/cirrhotic HCV patients P<0.001 Time (y) van der Meer AJ, et al. JAMA 2012;308:2584–93. Non-SVR SVR IFN: interferon Effect of Treatment Rates & Efficacy on Mortality Wedemeyer JVH 2014 Diagnosis & Treatment Rates Dore, Ward & Thursz JVH 2014 Effect of Treatment Rates & Efficacy on Prevalence Wedemeyer JVH 2014 Reduction in HCV Prevalence Through Treatment of IVDU Martin et al. J.Hep 2011 Patient Cascade 100 Public awareness Screening 80 60 Education in 1o care Treatment settings 40 20 Clinical expertise IFN-free Efficacy of new antivirals 0 People with HCV People People People Patients Cured Diagnosed with Diagnosed with Diagnosed with HCV HCV and HCV and Referred for Treated Treatment Where to deliver HCV Treatment Drug & Alcohol Clinics Prisons Primary Care Secondary Care Testing * Testing † Testing ‡ Testing ** Counselling * Counselling † Counselling ‡ Counselling ** Assessment ** Assessment ** Assessment ** Referral Treatment ** Treatment ** Treatment ** Lab Services ** Commissioned by NHSE * Provided by Drug & Alcohol services Commissioned by CCG ** Provided by Secondary Care Commissioned by PHE † Provided by Offender Health services Commissioned by Offender Health ‡ Provided by Primary Care services Summary • The majority of people with HCV in the UK are PWID • Most people with HCV remain undiagnosed • PEG/RBV is unpleasant, ineffective and occasionally dangerous • PEG/RBV is a barrier to patient being diagnosed and treated • New drug therapy is effective, safe and side effect free • HCV epidemic can be controlled if HCV is diagnosed and treated • Treatment as prevention is cost-effective • A high proportion of PWID have spells in prison • There are barriers to treating patients in prison / D&A centres • You cannot impose Peg-IFN therapy on patients Hepatitis C in the Drug using community The Hepatitis C Trust and Addaction 2014 • Elimination of hep C - in the drug using community • Drug service interventions – Workforce development, P2P education, Buddying • Bridging the gap between drug services and hospitals – Treatment in the community • Testing • Needle and syringe programmes What we know 216,000+ living with hepatitis C in the UK Over 100,000 diagnosed in England & Wales alone 90% of new infections from drug use Considerable drop out along the care pathway Large DNA rates after referral to specialist care Nottingham study (Irving et al., 2006) HCVAction survey estimates 30% attendance (Harris et al., 2012) MODELLING PROJECTIONS: CURRENT TREATMENT RATES INSUFFICIENT TO ACHIEVE OBSERVABLE DIFFERENCE IN HCV OVER 10 YEARS Martin NK, et al. MODELLING PROJECTIONS: ACHIEVABLE SCALE-UP WOULD MORE THAN HALVE HCV PREVALENCE WITH NEW DAAs IN 10 YEARS Martin NK, et al. ELIMINATION IN CORNWALL Currently treating 16 PWID of 2000 PWID Martin NK, et al. PWID: not a barrier to SVR in OST therapy H. Jafferbhoy J Vir Hep 2011 Supporting Interventions • Workforce development – Discuss hep C with confidence – Raise awareness, encourage safer practice – Increased testing – Better referral / pathway – Better support for people for HCV Workforce development P2P Education Raising awareness amongst service users • Myths or inaccurate information that persist within drug using communities. • Ensure PWIDs understand the realities of hepatitis C prevention, transmission, diagnosis, treatment and care. Hep C Trust P2P project • Since August 2010 • Spoken to 4000 peers • Visited 470 services • Continuous feedback: – Gary encouraged me to explore my options, and I have since – accessed specialist care Our survey said…. • 20 service evaluations after visit • 18 services said: – Between 1 – 5 tests after P2P visit • 7 services said: – At least one went onto treatment Buddying • Peers trained to offer support • Appointment companions • One to one visits (Coffee shop) • Text Reminder / Check in service Buddying Positive support between 2012 and 2013 • 185 hospital appointments for 104 service users • 36 of these people have been treated • Majority of the remainder are in the care pathway Up scaling the interventions • Drug and Alcohol services: – Staff training – Use existing network of volunteers – Use existing volunteer management procedures Mobilise P2P educators and Buddies Closing the Gap Closing the gap between drug services and hospital referral systems to ensure that patients make it from diagnosis through to treatment/monitoring if that is their wish. Fear of hospital visits – Unknown territory / Unknown staff Stigma – Rumours of not treating PWID Difficulty in navigating hospital admin systems Closing the Gap • DNA rates at hospitals post referral of PWID – 5 hospitals contacted, ALL experienced high DNA’s, some as high as 90% • Solution – Buddying – Treatment at the drug service The evidence is compelling • WSM Treating in the community! • Identified huge DNA with CCG • Part time Nurse comes to drug service runs clinic • Currently approx. 20 on treatment CURRENT HCV TREATMENT RATES AMONG PWID: 4-FOLD DIFFERENCE IN UK SITES PWID Population SITE Estimate (min, max) Bristol 3200 4400 East London 2400 6000 Manchester 2300 4000 Nottingham 1300 2500 Plymouth 110 2000 Tayside/Dundee 2000 3000 North Wales 1700 3400 HCV chronic prevalence (min, max) 37% 37% 48% 37% 30% 20% 27% 48% 48% 56% 44% 37% 27% 33% PWID HCV Rate per 1000 Treatments PWID per year (min, max) 18 25 63 32 17 34 18 4.1 4.2 15.8 12.8 8.5 11.3 5.3 5.6 10.4 27.4 24.6 15.5 17.0 10.6 Defined PWID as on OST or injected <3 years Martin NK, et al. Moving into DAA Treatment • Community • Offered alongside treatment methadone prescribing • Small upscale in treatment – big • Possibility of impact Directly Observed Therapy (DOT) • Treatment of PWID as prevention Testing • What is an offer? – SU often unable to attend testing next door – Testing at single point of contact (DBST) • What is a referral? – Why do drug services test only to send SU to GP for further referral and test? Testing • Scotland says: “If you can test or read a test result you can refer” • 81% of tests are carried out by support workers, without clinical qualifications • Less burden at GP surgeries NSP Aims Ensure adequate coverage of needles, syringes and paraphernalia: 100% coverage of Needle provision: • through pharmacies • at one stop drug services NSP Reality • Service users are encouraged to stick to prescribed OST and not use street drugs • Service user has to collect Needles from the same building as prescribing • Service user goes to pharmacy for Needles where no intervention is encouraged NSP • European NSP Guidance: “Obtaining the trust of the clients ... is essential for the prevention of infectious diseases” Many NSP’s are run by service users in Europe One day • DAA’s, The Cure will be available to all • Largely administered within Drug services – Possibly alongside OST (DOT) • Hep C will be eliminated • We need to lay the groundwork – Changing the peer message in the community – Better communication between services Call to Action • Educating Consultants on PWID – If not treating, why not? • Push for treating in the community – Encourage NHS Staff to leave the hospital • Treatment Benchmarks to meet our aims – How many do you need to treat to have impact Call to Action • Direct referrals should be encouraged • Peer networks utilised • Independent NSP “ANYONE SHOULD BE ABLE TO ACCESS TREATMENT IF THEY WISH – EVEN THOSE STILL INJECTING DRUGS OR ON OPIOID SUBSTITUTES” The Hepatitis C Trust [email protected]. uk 27 Crosby Row London, SE1 3YD www.hepctrust.org.uk