* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 19.7 Reversible Addition Reactions of Aldehydes and Ketones
Bottromycin wikipedia , lookup
Kinetic resolution wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Metal carbonyl wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Marcus theory wikipedia , lookup
Discodermolide wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Elias James Corey wikipedia , lookup
Hydrogenation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Stille reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
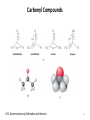
Organic Chemistry, 5th ed. Marc Loudon Chapter 19 The Chemistry of Aldehydes and Ketones. Carbonyl-Addition Reactions Eric J. Kantorowski California Polytechnic State University San Luis Obispo, CA Chapter 19 Overview • • • • • • • • • 19.1 Nomenclature of Aldehydes and Ketones 19.2 Physical Properties of Aldehydes and Ketones 19.3 Spectroscopy of Aldehydes and Ketones 19.4 Synthesis of Aldehydes and Ketones 19.5 Introduction to Aldehyde and Ketone Reactions 19.6 Basicity of Aldehydes and Ketones 19.7 Reversible Addition Reactions of Aldehydes and Ketones 19.8 Reduction of Aldehydes and Ketones to Alcohols 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents • 19.10 Acetals and Their Use of Protecting Groups 2 Chapter 19 Overview • • • • • 19.11 Reactions of Aldehydes and Ketones with Amines 19.12 Reduction of Carbonyl Groups to Methylene Groups 19.13 The Wittig Alkene Synthesis 19.14 Oxidation of Aldehydes to Carboxylic Acids 19.15 Manufacture and Use of Aldehydes and Ketones 3 Carbonyl Compounds • Aldehydes and ketones have the following general structure 19.1 Nomenclature of Aldehydes and Ketones 4 Carbonyl Compounds 19.1 Nomenclature of Aldehydes and Ketones 5 Common Nomenclature 19.1 Nomenclature of Aldehydes and Ketones 6 Prefixes Used in Common Nomenclature 19.1 Nomenclature of Aldehydes and Ketones 7 Common Nomenclature 19.1 Nomenclature of Aldehydes and Ketones 8 Substitutive Nomenclature 19.1 Nomenclature of Aldehydes and Ketones 9 Substitutive Nomenclature 19.1 Nomenclature of Aldehydes and Ketones 10 Physical Properties • Most simple aldehydes and ketones are liquids 19.2 Physical Properties of Aldehydes and Ketones 11 IR Spectroscopy • Strong C=O stretch: 1700 cm-1 19.3 Spectroscopy of Aldehydes and Ketones 12 IR Spectroscopy • Conjugation with a p bond lowers the absorption frequency 19.3 Spectroscopy of Aldehydes and Ketones 13 IR Spectroscopy • The C=O stretching frequency in small-ring ketones is affected by ring size 19.3 Spectroscopy of Aldehydes and Ketones 14 1H NMR Spectroscopy • The reason for the large d value for aldehydic protons is similar to that for vinylic protons • However, the electronegative O increases this shift farther downfield 19.3 Spectroscopy of Aldehydes and Ketones 15 13C NMR Spectroscopy • Aldehyde and ketone C=O: d 190-220 • a-Carbons: d 30-50 19.3 Spectroscopy of Aldehydes and Ketones 16 UV/Vis Spectroscopy • p → p*: 150 nm (out of the operating range) • n → p*: 260-290 nm (much weaker) 19.3 Spectroscopy of Aldehydes and Ketones 17 UV/Vis Spectroscopy 19.3 Spectroscopy of Aldehydes and Ketones 18 Mass Spectrometry 19.3 Spectroscopy of Aldehydes and Ketones 19 Mass Spectrometry • What accounts for the m/z = 58 peak? 19.3 Spectroscopy of Aldehydes and Ketones 20 Mass Spectrometry • The McLafferty rearrangement involves a hydrogen transfer via a transient sixmembered ring • There must be an available g-H 19.3 Spectroscopy of Aldehydes and Ketones 21 Summary of Aldehyde and Ketone Preparation 1. Oxidation of alcohols 2. Friedel-Crafts acylation 3. Hydration of alkynes 4. Hydroboration-oxidation of alkynes 5. Ozonolysis of alkenes 6. Periodate cleavage of glycols 19.4 Synthesis of Aldehydes and Ketones 22 Carbonyl-Group Reactions • Reactions with acids • Addition reactions • Oxidation of aldehydes 19.5 Introduction to Aldehyde and Ketone Reactions 23 Basicity of Aldehydes and Ketones • The carbonyl oxygen is weakly basic • One resonance contributor reveals that carbocation character exists • The conjugate acids of aldehydes and ketones may be viewed as a-hydroxy carbocations 19.6 Basicity of Aldehydes and Ketones 24 Basicity of Aldehydes and Ketones • a-hydroxy and a-alkoxy carbocations are significantly more stable than ordinary carbocations (by ~100 kJ mol-1) 19.6 Basicity of Aldehydes and Ketones 25 Addition Reactions • One of the most typical reactions of aldehydes and ketones is addition across the C=O 19.7 Reversible Addition Reactions of Aldehydes and Ketones 26 Mechanism of Carbonyl-Addition Reactions 19.7 Reversible Addition Reactions of Aldehydes and Ketones 27 Addition Reactions • The addition of a nucleophile to the carbonyl carbon is driven by the ability of oxygen to accept the unshared electron pair 19.7 Reversible Addition Reactions of Aldehydes and Ketones 28 Addition Reactions • The nucleophile attacks the unoccupied p* MO (LUMO) of the C=O 19.7 Reversible Addition Reactions of Aldehydes and Ketones 29 Addition Reactions • The second mechanism for carbonyl addition takes place under acidic conditions 19.7 Reversible Addition Reactions of Aldehydes and Ketones 30 Equilibria in Carbonyl-Addition Reactions • The equilibrium for a reversible addition depends strongly on the structure of the carbonyl compound 1. Addition is more favorable for aldehydes 2. Addition is more favorable if EN groups are near the C=O 3. Addition is less favorable when groups that donate electrons by resonance to the C=O are present 19.7 Reversible Addition Reactions of Aldehydes and Ketones 31 Equilibrium Constants for Hydration 19.7 Reversible Addition Reactions of Aldehydes and Ketones 32 Relative Carbonyl Stability 19.7 Reversible Addition Reactions of Aldehydes and Ketones 33 Carbonyl Stability • Any feature that stabilizes carbocations will impart greater stability to the carbonyl group • For example, alkyl groups stabilize carbocations more than hydrogens • Hence, alkyl groups will discourage addition reactions to the carbonyl group 19.7 Reversible Addition Reactions of Aldehydes and Ketones 34 Carbonyl Stability • Resonance can also add stability to the carbonyl group • However, EN groups make the addition reaction more favorable 19.7 Reversible Addition Reactions of Aldehydes and Ketones 35 Rates of Carbonyl-Addition Reactions • Relative rates can be predicted from equilibrium constants • Compounds with the most favorable addition equilibria tends to react most rapidly • General reactivity: formaldehyde > aldehydes > ketones 19.7 Reversible Addition Reactions of Aldehydes and Ketones 36 Reduction with LiAlH4 and NaBH4 19.8 Reduction of Aldehydes and Ketones to Alcohols 37 Reduction with LiAlH4 • LiAlH4 serves as a source of hydride ion (H:-) • LiAlH4 is very basic and reacts violently with water; anhydrous solvents are required 19.8 Reduction of Aldehydes and Ketones to Alcohols 38 Reduction with LiAlH4 • Like other strong bases, LiAlH4 is also a good nucleophile • Additionally, the Li+ ion is a built-in Lewis-acid 19.8 Reduction of Aldehydes and Ketones to Alcohols 39 Reduction with LiAlH4 • Each of the remaining hydrides become activated during the reaction 19.8 Reduction of Aldehydes and Ketones to Alcohols 40 Reduction with NaBH4 • Na+ is a weaker Lewis acid than Li+ requiring the use of protic solvents • Hydrogen bonding then serves to activate the carbonyl group 19.8 Reduction of Aldehydes and Ketones to Alcohols 41 Reduction with LiAlH4 and NaBH4 • Reactions by these and related reagents are referred to as hydride reductions • These reactions are further examples of nucleophilic addition 19.8 Reduction of Aldehydes and Ketones to Alcohols 42 Selectivity with LiAlH4 and NaBH4 • NaBH4 is less reactive and hence more selective than LiAlH4 • LiAlH4 reacts with alkyl halides, alkyl tosylates, and nitro groups, but NaBH4 does not 19.8 Reduction of Aldehydes and Ketones to Alcohols 43 Reduction by Catalytic Hydrogenation • Hydride reagents are more commonly used • However, catalytic hydrogenation is useful for selective reduction of alkenes 19.8 Reduction of Aldehydes and Ketones to Alcohols 44 Grignard Addition • Grignard reagents with carbonyl groups is the most important application of the Grignard reagent in organic chemistry 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 45 Grignard Addition • R-MgX reacts as a nucleophile; this group is also strongly basic behaving like a carbanion • The addition is irreversible due to this basicity 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 46 Organolithium and Acetylide Reagents • These reagents react with aldehydes and ketones analogous to Grignard reagents 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 47 Importance of the Grignard Addition • This reaction results in C-C bond formation • The synthetic possibilities are almost endless 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 48 Importance of the Grignard Addition 19.9 Reactions of Aldehydes and Ketones with Grignard and Related Reagents 49 Preparation and Hydrolysis of Acetals • Acetal: A compound in which two ether oxygens are bound to the same carbon 19.10 Acetals and Their Use of Protecting Groups 50 Preparation and Hydrolysis of Acetals • Use of a 1,2- or 1,3-diol leads to cyclic acetals • Only one equivalent of the diol is required 19.10 Acetals and Their Use of Protecting Groups 51 Preparation and Hydrolysis of Acetals 19.10 Acetals and Their Use of Protecting Groups 52 Preparation and Hydrolysis of Acetals • Acetal formation is reversible • The presence of acid and excess water allows acetals to revert to their carbonyl form • Acetals are stable in basic and neutral solution 19.10 Acetals and Their Use of Protecting Groups 53 Hemiacetals • Hemiacetals normally cannot be isolated • Exceptions include simple aldehydes and compounds than can form 5- and 6membered rings 19.10 Acetals and Their Use of Protecting Groups 54 Hemiacetals 19.10 Acetals and Their Use of Protecting Groups 55 Protecting Groups • A protecting group is a temporary chemical disguise for a functional group preventing it from reacting with certain reagents 19.10 Acetals and Their Use of Protecting Groups 56 Protecting Groups 19.10 Acetals and Their Use of Protecting Groups 57 Reactions with Primary Amines • Imines are sometimes called Schiff bases 19.11 Reactions of Aldehydes and Ketones with Amines 58 Reactions with Primary Amines • The dehydration of water is typically the ratelimiting step 19.11 Reactions of Aldehydes and Ketones with Amines 59 Derivatives • Before the advent of spectroscopy, aldehydes and ketones were characterized as derivatives 19.11 Reactions of Aldehydes and Ketones with Amines 60 Some Imine Derivatives 19.11 Reactions of Aldehydes and Ketones with Amines 61 Reactions with Secondary Amines • Like imine formation, enamine formation is reversible 19.11 Reactions of Aldehydes and Ketones with Amines 62 Reactions with Secondary Amines 19.11 Reactions of Aldehydes and Ketones with Amines 63 Reactions with Tertiary Amines • Tertiary amines do not react with aldehydes or ketones to form stable derivatives • They are good nucleophiles, but the lack of an N-H prevents conversion to a stable compound 19.11 Reactions of Aldehydes and Ketones with Amines 64 Reduction of Aldehydes and Ketones • Complete reduction to a methylene (-CH2-) group is possible by two different methods • Wolff-Kishner reduction: 19.12 Reduction of Carbonyl Groups to Methylene Groups 65 Reduction of Aldehydes and Ketones • The Wolff-Kishner reduction takes place under highly basic conditions • It is an extension of imine formation 19.12 Reduction of Carbonyl Groups to Methylene Groups 66 Reduction of Aldehydes and Ketones • Clemmensen reduction: • This reduction occurs under acidic conditions • The mechanism is uncertain 19.12 Reduction of Carbonyl Groups to Methylene Groups 67 The Wittig Alkene Synthesis • This reaction is completely regioselective, assuring the location of the alkene 19.13 The Wittig Alkene Synthesis 68 The Wittig Alkene Synthesis • Occurs via an addition-elimination sequence using a phosphorous ylide • An ylid (or ylide) is any compound with opposite charges on adjacent, covalently bound atoms 19.13 The Wittig Alkene Synthesis 69 The Wittig Alkene Synthesis 19.13 The Wittig Alkene Synthesis 70 Preparation of the Wittig Reagent • Any alkyl halide that readily participates in SN2 reactions can be used 19.13 The Wittig Alkene Synthesis 71 The Wittig Alkene Synthesis • Retrosynthetically • Stereochemistry 19.13 The Wittig Alkene Synthesis 72 Carboxylic Acids from Aldehydes • The hydrate is the species oxidized 19.14 Oxidation of Aldehydes to Carboxylic Acids 73 Carboxylic Acids from Aldehydes • This is known as the Tollen’s test • A positive indicator for an aldehyde is the deposition of a metallic silver mirror on the walls of the reaction flask 19.14 Oxidation of Aldehydes to Carboxylic Acids 74 Production and Use of Aldehydes • The most important commercial aldehyde is formaldehyde • Its most important use is in the synthesis of phenol-formaldehyde resins 19.15 Manufacture and Use of Aldehydes and Ketones 75 Production and Use of Ketones • The most important commercial ketone is acetone • It is co-produced with phenol by the autoxidation-rearrangement of cumene 19.15 Manufacture and Use of Aldehydes and Ketones 76