* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Current drugs for weight loss
Survey
Document related concepts
Transcript
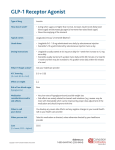
Short report Current drugs for weight loss Orlistat is the only weight loss drug currently licensed in the UK. Outside the UK, there are now four other agents approved for weight loss. Dr Kate Millar and Dr Ruth Poole here review the evidence regarding the comparative efficacy and adverse effects of the five different drug treatments. Background According to WHO data, the global prevalence of obesity has doubled between 1980 and 2014. By 2014, 39% of the world’s adults (1.9 billion indiv iduals) were overweight and 18% were obese (600 million individuals).1 As well as being a major risk factor for type 2 diabetes (T2DM), overweight and obesity contribute to morbidity and mortality from cardiovascular dis ease, osteoarthritis and some cancers such as endometrial, breast and colon. Excess weight now leads to more deaths than malnutrition worldwide.1 The reasons for this increase are complex. Easy access to energy rich foods and decreased activity have been recog nised for many years2 but more recent evidence suggests that gut microbia, epigenetic mechanisms, increasing maternal age, greater fecundity among people with higher adiposity, and endo crine disrupting chemicals may all be having some effect.3 Lifestyle modifications are gener ally ineffective for weight loss in the long term due to the body’s adapta tions to lower energy expenditure in response to reduced calorific intake and the high levels of motivation needed to sustain significant changes. Even in clinical studies where patients are highly motivated, initial weight loss is followed by gradual weight regain.4 Equally, bariatric surgery is invasive and potentially dangerous. As more research has allowed a greater understanding of the pathways and signalling involved in regulating metabolism and appetite, this has fuelled interest into new potential therapeutic targets. The use of phar macological agents for weight loss has historically been associated with sig nificant side effect burden and safety concerns. Fenfluramine, a serotonin increasing anorectic, was withdrawn from the market in 1997 after being PRACTICAL DIABETES VOL. 33 NO. 7 shown to cause potentially fatal pul monary hypertension and cardiac valvulopathy. Rimonabant, a selective cannabinoid receptor blocker, was withdrawn in 2008 because of serious psychiatric side effects including risk of suicide. Sibutramine, a serotonin– norepinephrine reuptake inhibitor, was withdrawn in 2010 because of its association with increased cardiovas cular events and strokes. Orlistat is the only weight loss drug currently licensed in the UK. It works by inhibiting pancreatic lipases and thereby decreasing dietary fat absorp tion and increasing faecal fat excre tion. Weight loss in the first year of treatment is around 5–10kg,5 and this has been shown to be maintained over four years with continued use.6 Orlistat reduces the risk of developing diabetes in obese patients with impaired glucose tolerance by around a third.6 In patients with diabetes, orlistat has been demonstrated to reduce HbA1c by 0.6% (7mmol/mol) over 52 weeks.7 It has also been shown to improve blood pressure and serum lipids beyond expected levels from weight reduction alone.8,9 Between 15–30% of patients experience gastrointestinal (GI) side effects associated with orlistat; generally these improve as patients learn to avoid high fat diets. Absorption rates of fat soluble vitamins A, D, E and K are lowered with orlistat therapy (vitamin D in particular) and supplements should be considered. Here we review four new weight loss drugs: lorcasarin (Belviq), high dose liraglutide (Saxenda), naltrexone/ bupropion (Contrave or Mysimba) and phentermine/topiramate (Qsymia). We review their efficacy and their adverse effects. Saxenda is adminis tered by subcutaneous injection while Belviq, Contrave and Qsymia are all oral tablets. or obstructive sleep apnoea. It is not currently licensed in the UK. A multicentre placebo-controlled trial of lorcaserin found that at one year 47.5% of patients in the lorcaserin group had lost 5% or more of their body weight compared to 20.3% in the placebo group (p<0.001) This corre sponds to a 3–4kg difference in weight loss between the two groups. They also showed that in year two weight loss was more likely to be maintained if they continued on lorcaserin rather than switching to placebo.10 A trial looking at the efficacy of lorcaserin for weight loss in patients with T2DM randomised patients to lorcaserin 10mg once a day, 10mg twice a day or placebo. Patients were being treated with metformin, a sulphonyl urea or both. At one year, there was no difference in weight loss between the two active treatment groups, but both had lost three times more weight than the placebo group (6% vs 2%) and significantly more patients lost more than 5% of their body weight on lorcaserin (44.7% once-daily dose and 37.5% twice-daily dose) compared to placebo (16.1%). There was also a sig nificant improvement in glycaemic control with HbA1c decreasing 0.9% to 1.0% (10–11mmol/mol) in the lorca serin groups and 0.4% (4mmol/mol) in the placebo group.11 Adverse effects of lorcaserin are generally mild with headache, back pain and naso-pharyngitis occurring with greater frequency than compared to the placebo group. Neuropsychiatric effects were not significantly increased in any of the trials. With regard to concerns of serotonin-associated valvu lopathy, pooled analysis of the phase 3 trials showed that the rates of FDA defined valvulopathy were 2.3% and 2.2% for patients taking lorcaserin or placebo respectively.12 Lorcaserin (Belviq) Lorcaserin is a selective agonist of the serotonin 2C receptor which reduces food intake by increasing satiety. It was approved by the US Food and Drug Administration (FDA) in 2012 as an adjunct to lifestyle interventions for patients with BMI above 30kg/m2 or above 27kg/m2 with at least one medical comorbidity such as T2DM Liraglutide (Saxenda) Liraglutide is an analogue of the incretin hormone glucagon-like pep tide 1 (GLP-1) and has dual thera peutic benefits in terms of glycaemic control and weight loss due to enhanced postprandial insulin secre tion, delayed gastric emptying and decreased food intake. Liraglutide is established in Europe and America COPYRIGHT © 2016 JOHN WILEY & SONS 229 Short report Current drugs for weight loss as a treatment option for patients with T2DM with BMI above 30kg/m2 or above 27kg/m2 with at least one weight-related comorbidity at doses of 0.6mg to 1.8mg daily, and is linked to a significant reduction in weight (2–3kg) when compared to placebo or glimepiride.13 In the UK, liraglu tide can only be prescribed for patients with a BMI above 35kg/m2 unless there are comorbidities, and the maximum dose is 1.2mg daily.14 High-dose liraglutide (3mg) has been licensed as a treatment for weight loss in people without diabe tes in the US since September 2014 under the trade name Saxenda, but is not yet licensed for this indication in the UK. There is evidence that liraglutide, at the doses licensed for patients with diabetes, is being used in the UK for obese patients without dia betes. Twenty-two obese individuals without diabetes attending a special ist obesity service were enrolled in a 12-month study to see if liraglutide 1.8mg could avoid bariatric surgery. Fourteen completed the study with a mean weight loss of 12.1kg. Two participants withdrew because of lack of efficacy, two because of GI side effects and the rest failed to complete the study as their primary care funding of the treat ment was withdrawn.15 Other studies of the efficacy of liraglutide for inducing weight loss in those without diabetes have used higher doses. In a 56-week dou ble-blind trial, 63.2% of patients receiving 3mg liraglutide lost at least 5% of their body weight compared to 27.1% in the placebo group. Additionally, HbA1c, quality of life and cardio-metabolic risk factors all improved.16 A randomised con trolled trial comparing the efficacy of liraglutide with that of orlistat showed that patients taking the two highest doses of liraglutide (2.4 and 3.0mg) lost significantly more weight than those taking orlistat (6.3, 7.2 and 4.1kg, respectively).17 The SCALE diabetes trial looked at the efficacy and safety of the higher liraglutide 3.0mg dose in patients with T2DM and found that at 56 weeks weight loss was 6.0% (6.4kg) with 3.0mg dose, 4.7% (5.0kg) with 1.8mg dose and 2% (2.2kg) with placebo. HbA1c significantly improved 230 PRACTICAL DIABETES VOL. 33 NO. 7 compared to placebo with treatment difference of -0.93% (10mmol/mol) in the 3.0mg dose group. Mean systolic blood pressure was signifi cantly decreased (treatment differ ence -2.6mmHg) in the liraglutide dose compared to placebo without a dose effect. Liraglutide 3.0mg also significantly improved levels of total cholesterol, VLDL, HDL and triglyc erides compared with placebo. Liraglutide was associated with a mean heart rate increase of 2.0 beats per minute compared to a reduction of 1.4 beats per minute for placebo. Given the improvements in the other cardiovascular risk factors, the long-term clinical relevance of this is unclear.18 The higher dosages of liraglutide are, however, associated with a higher incidence of GI side effects. The 3mg dose caused GI side effects in 77% of participants and was the most com mon cause for cessation of treat ment. There were eight people in the liraglutide groups who withdrew (2.2%) because of nausea, and five (1.3%; in the 2.4mg and 3.0mg groups) because of vomiting. Nobody in the placebo or orlistat groups withdrew because of such events.17 Naltrexone/bupropion (Contrave/Mysimba) The combination of naltrexone and bupropion was approved by the FDA in September 2014 under the trade name Contrave and in some European countries in March 2016 as Mysimba. It is not currently licensed in the UK. Bupropion stim ulates hypothalamic proopiomel anocortin (POMC) neurons while naltrexone blocks opioid-mediated POMC neurone auto-inhibition, inducing satiety. Given their estab lished uses in addiction, it is also postulated that this combination may help modulate central nervous system reward pathways. Efficacy and safety of naltrexone/ bupropion combination therapy were tested in the Contrave Obesity Research (COR) studies. COR-I com pared two different doses of naltrex one (16 and 32mg) combined with bupropion against placebo in 1742 participants.19 COR-II compared naltrexone/bupropion 32/360mg against placebo in 1496 partici pants.20 Primary endpoints in each trial were percentage weight loss and proportion of subjects achieving more than 5% weight loss. All part icipants were either obese (BMI 30–45kg/m2) or overweight (27– 45kg/m2) with dyslipidemia and/or hypertension. Both trials were con ducted over 56 weeks. In COR-1, 48% of participants assigned to naltrexone 32mg plus bupropion had a decrease in body weight of 5% or more compared with 16% of participants assigned to placebo. Mean reduction in body weight was 6.1% in the naltrexone 32mg plus bupropion group versus 1.3% in the placebo group.19 Similar results were seen in COR-II with a 6.1% weight reduction by week 56 in the naltrexone/bupropion group against 1.2% in the placebo group, and 50.5% achieved a 5% weight loss compared to 17.1% of participants on placebo.20 Side effects were common, par ticularly nausea, headache and con stipation. Drop-out rates were high with only 54% of participants in COR-II completing the study. In the active treatment arm, this was due to side effects while, in the placebo arm, the same proportion of patients withdrew due to insufficient weight loss.20 Pulse and blood pressure were not adversely affected. The LIGHT study was conducted to establish the cardiovascular safety of Contrave. More than 8000 partici pants were randomised either to naltrexone/bupropion or to placebo. However, the trial was stopped early after public release of confidential interim data by the sponsor suggesting a 40% reduction in major cardiovascu lar events in the naltrexone/bupro pion group.21 This was not confirmed after further analysis, but cardiovascu lar safety was not established and further evaluation is required. Phentermine/topiramate (Qsymia) Phentermine is a centrally-acting sym pathomimetic and acts as an appetite suppressant. As a single agent it is the most frequently prescribed drug for weight loss in the US where it is approved only for short-term (up to 12 weeks) use because of its addictive potential. Topiramate is an anti-epi leptic drug, also licensed for migraine prophylaxis which has been noted to COPYRIGHT © 2016 JOHN WILEY & SONS Short report Current drugs for weight loss Weight loss treatment Current UK licence Mode of action Weight loss potential Metabolic benefits Side effects Orlistat6,27 Yes Inhibition of pancreatic lipase; decreased absorption of ingested fat 10.2% over 1 year Reductions in total and LDL cholesterol Gastrointestinal Lorcaserin10 No Selective serotonin agonist; increased satiety; reduced food intake 5.8% over 1 year Reduction in HbA1c in patients with diabetes Headache, back pain and naso-pharyngitis Liraglutide16,18,26 No GLP-1 agonist; increased satiety; reduced food intake 8% over 56 weeks Reduction in HbA1c in patients with diabetes; reduction in blood pressure and improved lipid profile Gastrointestinal Naltrexone/ bupropion19 No POMC (proopiomelanocortin) modulation; increased satiety; reduced food intake 6.1% over 56 weeks – Nausea, headache and constipation Phentermine/ topiramate22 No Centrally-acting sympathomimetic; reduced appetite; reduced food intake 9.8% over 56 weeks HbA1c in patients with diabetes; reduction in blood pressure Gastrointestinal, neurological and psychiatric Table 1. Comparison of currently available weight loss treatments have some weight loss effects. Qsymia was approved as a combination treat ment in the US in 2012 but is not licensed in the UK. The benefits of low-dose Qsymia were assessed in the CONQUER trial. A total of 2487 patients were randomised to phentermine/ topiramate at either 7.5/46mg or 15/92mg daily or to placebo; 20% of participants had T2DM at study entry. At 56 weeks, participants tak ing Qsymia had lost 8–10kg while those on placebo had lost 1.4kg. Five percent weight loss was achieved by 62% and 70% of participants in the two active arms and in 21% of partic ipants taking placebo. There was a mean decrease in systolic blood pressure of 2.3mmHg compared to placebo in the 7.5/46mg dose and 3.2mmHg in the 15/92mg group following one year of treatment. Among the 388 subjects with T2DM, reductions in HbA1c from baseline were 0.1% (1mmol/mol) for pla cebo compared to 0.4% (4mmol/ mol) with both doses of Qsymia.22 The SEQUEL trial was an exten sion of the CONQUER trial with par ticipants continuing on the same dose of Qsymia or placebo for a further 52 weeks. Weight loss during the first 56 weeks in the Qsymia group was maintained over a further 52 weeks. Blood pressure was not different between the groups although 13.1% and 15.6% of patients in the two PRACTICAL DIABETES VOL. 33 NO. 7 active treatment groups had reduc tions in their antihypertensive medi cations while 11% of participants taking placebo had their antihyper tensive medications increased.23 The most common adverse effects were dry mouth, constipation and paraesthesia as well as a dose-related increase in incidence of depression, anxiety and attention difficulties. This is further underlined as a study exam ining its use in the real world environ ment of a multidisciplinary weight loss clinic noted an adverse event cessation rate of 40% with neurologi cal side effects predominating.24 Discussion As the prevalence of obesity and related comorbidities continues to climb, pharmacological interventions for weight loss remain limited. Overall, the usefulness of weight loss medications are limited by side effects which result in high drop-out rates in medical trials as well as poor compliance in real world prescribing. In a population-based cohort, at one year after prescription of orlistat or sibutramine, less than 10% of patients were still on their medication and by two years only 2% continued on either medication.25 A further diffi culty is that weight loss tends to slow and then plateau with continued treatment and the majority of patients regain weight when their weight loss drugs are stopped. However, it is of interest that trials have shown that a good initial response predicts longterm response and this underlines not only the importance of assessing responses within six months and discontinuing treatment in those with no measurable benefit, but also the potential value of further investigat ing the characteristics/phenotype associated with a good response. This may allow for more personalised treatments in the future. Belviq, Saxenda, Contrave and Qsymia have all been shown to be effective for weight management with the potential for weight loss of between 5.8–9.8% of starting weight over the first year of treatment (see Table 1). However, none are more effective than orlistat. There is also at this stage a lack of long-term data (more than two years) available for the new medications discussed in contrast to the established long-term safety profile of orlistat. These new weight loss drugs need to be considered with caution. Each has demonstrated significant bene fits for weight reduction but none is without significant side effects. Weight loss alone may be beneficial for patient self-esteem and for reduction in weight-related joint disease and thrombo-embolic risk. An ideal drug would also reduce the metabolic complications of obesity including glycaemia, blood pressure and lipid profile and demonstrate COPYRIGHT © 2016 JOHN WILEY & SONS 231 Short report Current drugs for weight loss significant reductions in cardiovascu lar endpoints. At this stage, some of the new weight loss treatments have demonstrated reductions in HbA1c, blood pressure and cholesterol (see Table 1), but until recently none have shown reduction in myocardial infarction (MI), stroke or mortality. This has changed with the recent publication of the LEADER trial. In all, 9340 patients with T2DM and high cardiovascular risk were ran domised to 1.8mg of liraglutide or placebo. Over 3.8 years of follow up, the primary outcome of death from cardiovascular disease, non-fatal MI or non-fatal stroke was 13% in the liraglutide arm and 14.9% in the placebo arm. Cardiovascular mortal ity was lower in the liraglutide group than in the placebo group (4.7% vs 6.0%) as was total mortality (8.2% vs 9.6%). Differences in non-fatal MI and stroke did not reach statistical significance.26 Arguably the most exciting and promising line of new pharmacolog ical treatments are those targeted at gut hormones, given that research has proven their role in regulating appetite, metabolism, gut motility, secretion and even acting as neuro transmitters. GLP-1 analogues have paved the way towards developing gut hormones as therapeutics but have limited efficacy and dosedepend ent side effects. However, other gut hormone pathways are also yet to be exploited and new combi nations of hormone analogues are currently being developed. This includes peptide YY and pancreatic polypeptide analogues which are currently in phase 1 trials and hold potential to deliver better appetite suppression with fewer dose-depend ent side effects. Furthermore, the use of agents to increase energy expenditure alongside appetite sup pression is also under development which would represent a big step forward given that the body normally counter regulates weight loss by reducing energy expenditure and thus limiting weight loss. The poten tial use of glucagon receptor agonists in combination with GLP-1 in this way could therefore lead to a much greater weight loss than either pep tide could achieve alone. New com binations of gut hormone analogues could thus mimic physiological 232 PRACTICAL DIABETES VOL. 33 NO. 7 processes to provide safe weight loss at a comparable level to surgery and this represents an exciting area of investigation for pharmacological agents in the future. Conclusion In the UK, orlistat remains the only licensed weight reduction treatment although, for patients with T2DM, GLP-1 agonists such as liraglutide already used for treatment of hyper glycaemia also benefit patients in terms of weight loss. Whether the increased side effects with the higher dose as Saxenda will outweigh the additional weight loss benefits in clinical practice remains to be seen. The possibility of alternative oral medications such as Belviq, Contrave or Qsymia in the UK will depend on future studies of long-term efficacy and any long-term harms found during real world prescribing in the US and Europe. Kate Millar, MBChB, MRCP, Speciality Registrar, Royal Hampshire County Hospital, Winchester, UK Ruth Poole, DM, FRCP, Consultant, Poole Hospital NHS Foundation Trust, Poole, UK Correspondence to: Dr Ruth Poole, Poole Hospital NHS Foundation Trust, Poole BH15 2JB, UK; email: [email protected] Declaration of interests There are no conflicts of interest declared. References 1. World Health Organisation Fact Sheet 311, Jan 2015. www.who.int/mediacentre/factsheets/fs311/en/. 2. Prentice AM, Jebb SA. Obesity in Britain: gluttony or sloth? BMJ 1995;311(7002):437–9. 3. McAllister EJ, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009; 49(10):868–913. 4. Dansinger ML, et al. Meta-analysis: The effect of dietary counseling for weight loss. Ann Intern Med 2007;147(1):41–50. 5. Leblanc ES, et al. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Intern Med 2011;155(7):434–47. 6. Torgerson JS, et al. Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27(1):155–61. 7. Kelley DE, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: A 1-year randomized controlled trial. Diabetes Care 2002;25(6):1033–41. 8. Siebenhofer A, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev 2013;3:CD007654. 9. Tonstad S, et al. The effect of the gastrointestinal lipase inhibitor, orlistat, on serum lipids and lipoproteins in patients with primary hyperlipidaemia. Eur J Clin Pharmacol 1994;46(5):405–10. 10. Smith SR, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363(3):245–56. 11. O’Neil PM, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20(7):1426–36. 12. Aronne L, et al. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med 2014;126(6):7–18. 13. Nauck M et al.; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32(1):84–90. 14.NICE technology appraisal guidance [TA203]. Liraglutide for the treatment of type 2 diabetes mellitus. NICE, 27 October 2010. 15. Gillani SMR, Singh BM. The use of GLP-1 agonist therapy, liraglutide, is associated with significant weight loss in morbidly obese people without diabetes. Br J Diab Vasc Dis 2015;15(2):61–4. 16. Pi-Sunyer X, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0mg of liraglutide in weight management. N Engl J Med 2015;373(1):11–22. 17. Astrup A, et al.; NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374(9701):1606–16. 18. Davies MJ, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE diabetes randomized clinical trial. JAMA 2015;314(7):687–99. 19. Greenway FL, et al.; COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376(9741):595–605. 20. Apovian CM, et al.; the COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21(5):935–43. 21. Nissen SE, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors. JAMA 2016;315(10):990–1004. 22. Gadde KM, et al. Effects of low-dose, controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377(9774):1341–52. 23. Garvey WT, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95(2):297–308. 24.Neoh SL, et al. Combination phentermine and topiramate for weight maintenance: the first Australian experience. Med J Aust 2014;201:224–6. 25. Padwal R, et al. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes (Lond) 2007;31(10):1567–70. 26.Marso SP, et al.; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375(4):311–22. 27. Sjöström L, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998; 352(9123):167–72. COPYRIGHT © 2016 JOHN WILEY & SONS