* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Trulance™ - plecanatide
Survey
Document related concepts
Transcript
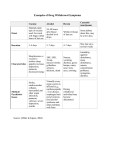
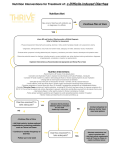
New Drug Introduction: Trulance / plecanatide Pharmacology Manufacturer Approval Date Indication Contraindications Black Box Warnings Warnings/ Precautions Pregnancy Lactation Pharmacokinetics Drug Interactions – Object/Precipitant Drug Adverse Effects (plecanatide) [placebo] Monitoring Efficacy Monitoring Toxicity Dosing Dosing – Maximum Renal Adjustment Hepatic Adjustment Administration Trulance™, plecanatide, is structurally related to human uroguanylin. It functions as a guanylate cyclase-C agonist that acts locally on the luminal surface of the intestinal epithelium. Synergy Pharmaceuticals Inc. January 19, 2017 Treatment of chronic idiopathic constipation (CIC) in adults Patients less than 6 years of age due to the risk of serious dehydration Patients with known or suspected mechanical gastrointestinal obstruction Risk of serious dehydration in pediatric patients Diarrhea: Patients may experience severe diarrhea. If severe diarrhea occurs, suspend dosing and rehydrate the patient. Category B – Available data on use in pregnant women is not sufficient to inform any drug-associated risks for major birth defects and miscarriage. Animal developmental studies observed no risk on embryo-fetal development with oral administration. Lactation Recommendation: No information available A – Minimally absorbed; AUC and Cmax cannot be calculated D – Plecanatide concentrations following clinically relevant doses are not measurable, thus, plecanatide is expected to be minimally distributed in tissues M – Metabolized in the GI tract to an active metabolite by low of the terminal leucine moiety; both plecanatide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids E – No excretion studies have been conducted in humans; plecanatide and its active metabolite are not measurable in plasma following administration of the recommended clinical doses. Neither plecanatide nor its active metabolite inhibited the CYP P450 enzymes 2C9 and 3A4, and they did not induce CYP3A4 in vitro. Plecanatide and its active metabolite are neither substrates nor inhibitor of the transporters P-gp or BCRP in vitro. Diarrhea (5%) [1%] Frequency of bowel movements No findings of toxicity observed in study 3 mg PO once daily The maximum recommended human dose is approximately 0.05 mg/kg/day, based on a 60-kg body weight. No adjustment necessary No adjustment necessary Take with or without food. Swallow tablets whole. For adult patients with swallowing difficulties, Trulance™ tablets can be crushed and administered either in applesauce or with water or administered with water via a nasogastric or gastric feeding tube. The use of crushed tablets has not been tested. Cost: Lexicomp: Accessed 2/22/2016 Dose(s) Brandor – Generic Trulance™ - plecanatide LinzessTM - linaclotide AmitizaTM – lubiprostone ZelnormTM – tegaserod 3 mg tablet 145 mcg capsule; 290 mcg capsule 8 mcg capsule; 24 mcg capsule 2 mg tablet; 6 mg tablet $ (30 day supply) Not yet available $388 $396 $148 Summary Trulance™, plecanatide, is a guanylate cyclase-C agonist indicated in adults for the treatment of chronic idiopathic constipation (CIC) Plecanatide is dosed by mouth as a once daily 3 mg tablet, which can be taken without regard to food The most common adverse effect is diarrhea, which typically occurs within 4 weeks of treatment initiation. If severe diarrhea occurs, suspend dosing and rehydrate the patient Plecanatide contains a black box warning for risk of serious dehydration in pediatric patients. For this reason, it is contraindicated in patients less than 6 years old. The safety and effectiveness of plecanatide have not been established in patients less than 18 years old. Plecanatide provides a new option for patients with CIC who have not found relief in other medications References: 1. www.trulancehcp.com 2. Trulance (plecanatide) [prescribing information]. New York, NY: Synergy Pharmaceuticals Inc.; January 2017. 3. Miner PB Jr, Koltun WD, Wiener GJ, De La Portilla M, Prieto B, Shailubhai K, Layton MB, Barrow L, Magnus L, Griffin PH. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017. doi: 10.1038/ajg.2015.611. [Epub ahead of print] Date Prepared: 2/22/2016 Editor: Peter G. Koval, Pharm.D., BCPS Author: Kristin Aloi, Pharm.D. Candidate, UNC Eshelman School of Pharmacy