* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1 - Max Stanley Chartrand, Ph.D.
Auditory processing disorder wikipedia , lookup
Public health genomics wikipedia , lookup
Telecommunications relay service wikipedia , lookup
Auditory system wikipedia , lookup
Lip reading wikipedia , lookup
Otitis media wikipedia , lookup
Auditory brainstem response wikipedia , lookup
Hearing loss wikipedia , lookup
Audiology and hearing health professionals in developed and developing countries wikipedia , lookup
The Relationship between External Ear Keratin Status
and Success in Physical Adaptation to Wearing Hearing Aids
A dissertation submitted to the
graduate faculty of the Department of Psychology
in partial fulfillment of the requirements for the degree of
DOCTOR OF PHILOSOPHY
by
Max Stanley Chartrand
Prescott, Arizona
December 29, 2006
ABSTRACT
The relationship between the health status of external auditory canal (EAC)
corneum stratum or keratin and success in the adaptation to wearing hearing aids and
earmolds was investigated. In a retrospective study the file data of 98 participants
(N=98, mean age=70.3 years) meeting the candidacy criteria were analyzed in terms of
successful adaptation to wearing hearing aids. Participant data were analyzed for
correlation between predictor and criterion variables. While age seemed to have no
significant relationship with keratin status (r = .089), sub-criterion variables of remakes
and returns for credit demonstrated an inverse relationship with the thickness of EAC
keratin (r = -.372 and r= -.386, respectively). Furthermore, keratin status and adaptation
demonstrated a strong correlational relationship (r = .627) over the 45-day fitting
process. Consequently it was demonstrated that a significant reduction in factors that
contribute to failure to fit may be realized by paying closer attention to the physiological
behavior of the human EAC and the underlying health dynamics that require
accommodation for each individual hearing aid user.
ii
ACKNOWLEDGMENTS
This project is actually the culmination of about 20 years’ work beginning when
I first embarked upon studying the little understood neuroreflexes of the human external
auditory canal (EAC). From that time forward, I’ve enjoyed the assistance of various
others who helped me find the missing links that eventually resulted in models of
complete reflex arcs, and discovery of underlying EAC physiology and immunology.
Hence, individuals such as M. Duncan McAllister, Ph.D., Ernest Zelnick, Ph.D., and Roy
F. Sullivan, Ph.D., all pioneers in their respective areas of interest, played important
roles in directing my attention to more productive interests. Robert Oliviera, Ph.D.
opened a view into the dynamicism of the human ear canal, while Wayne J. Staab,
Ph.D. provided glimpses into mapping out the epithelial territory of the EAC.
More recently, certain professors have inspired me to go forward with this
project. Heading the list would be the esteemed and noted researcher Kenna
Stephenson, M.D., who suggested that I pursue formal investigation into EAC keratin
and related issues of physiological behaviors. Nora Young, Ph.D. helped me look
deeper into causal factors—pharmacological side-effects, etc.—while my dissertation
committee chair, Robert Haussmann, Ph.D., provided invaluable insights into
quantifying that which is inherently qualitative. Certainly my experience in nearly four
years full-time in the program at Northcentral University has contributed mightily in
bringing about this culmination of something that has been of keen interest to me
personally and professionally for at least the past two decades.
Finally and foremost, I must give credit to Glenys Anne Chartrand, OTR, my
other half and most enthusiastic cheer leader. For it was she that supported my burning
iii
of midnight oil, arduous forays into uncharted waters, compilation of difficult-to-find
reference materials, and writing and rewriting until a workable contribution was
produced. It is my hope that the end product will change the way the hearing health
industry, indeed all of healthcare, looks at and understands the neurophysiology of the
human ear.
iv
TABLE OF CONTENTS
ABSTRACT ......................................................................................................................ii
ACKNOWLEDGMENTS .................................................................................................. iii
TABLE OF FIGURES ................................................................................................... viiii
LIST OF TABLES……………………………………………………………………………….ix
CHAPTER ONE .............................................................................................................. 1
INTRODUCTION .......................................................................................................... 1
Overview ................................................................................................................... 1
Statement of Problem................................................................................................ 2
Definition of Key Terms ............................................................................................. 3
Brief Review of the Literature .................................................................................... 7
Statement of Purpose.............................................................................................. 10
Research Expectations ........................................................................................... 11
CHAPTER TWO ............................................................................................................ 13
REVIEW OF THE LITERATURE ................................................................................ 13
Failure to Fit Deters Hearing Impaired Consumers ................................................. 13
Self-Assessment Studies and Reasons for Failure to Fit ........................................ 17
Elasticity and Dynamicism of the Human External Ear Canal ................................. 19
The Role of Keratin in the External Ear Canal ......................................................... 21
Mechanoreceptors of the External Auditory Canal .................................................. 26
Vagus (Arnold’s) reflex involvement in the External Auditory Canal. .................... 30
Trigeminal (Red) reflex and its effect during otoscopy, impression taking, and
when wearing hearing aids. .................................................................................. 33
Lymphatic (swelling) reflex. .................................................................................. 36
v
Effects of Medically Treatable Conditions on Physical Adaptation to Hearing Aids . 37
CHAPTER THREE ........................................................................................................ 45
METHODOLOGY ....................................................................................................... 45
Restatement of the Problem .................................................................................... 45
Statement of the Hypothesis ................................................................................... 47
Operational Definition of Variables .......................................................................... 49
Participants ............................................................................................................. 51
Materials .................................................................................................................. 51
Procedures and Measures ...................................................................................... 53
Data Processing. ..................................................................................................... 56
Methodological Assumptions and Limitations .......................................................... 57
Ethical Considerations............................................................................................. 59
CHAPTER FOUR .......................................................................................................... 63
RESULTS...................................................................................................................... 63
Overview .................................................................................................................... 63
Description of Participants .......................................................................................... 64
Participant Fitting Data ............................................................................................... 66
Participant Fitting Experience Data ............................................................................ 69
Keratin Status Data .................................................................................................... 70
Adaptation Experience Ratings .................................................................................. 73
Relationship Between Keratin and Adaptation ........................................................... 77
CHAPTER FIVE ............................................................................................................ 80
DISCUSSION ................................................................................................................ 80
vi
Summary of Findings ................................................................................................. 80
Explanation of the Findings ........................................................................................ 81
External Auditory Meatal Keratin Influence ................................................................ 85
Use of Video Otoscopy............................................................................................... 91
Internal & External Validity Issues .............................................................................. 93
Implications and Need for Future Research ............................................................... 98
Conclusion ............................................................................................................... 103
REFERENCES ............................................................................................................ 105
APPENDIX A ............................................................................................................... 126
Participant Data Sheet ............................................................................................. 126
APPENDIX B ............................................................................................................... 127
Keratin/Adaptation Rating Scale............................................................................... 127
APPENDIX C .............................................................................................................. 128
HIPAA Informed Consent Form ................................................................................ 128
APPENDIX D .............................................................................................................. 129
HEARING HEALTHCARE PROFESSIONAL AUTHORIZATION ............................. 129
APPENDIX E ............................................................................................................... 132
DATA FROM KERATIN/ADAPTATION RATING FORMS ....................................... 132
APPENDIX F ............................................................................................................... 135
SPSS CALCULATIONS ............................................................................................. 63
APPENDIX G………………………………………………………………………………. . 140
VIDEO OTOSCOPIC VIEWS & NOTES………………………………………………. 140
vii
TABLE OF FIGURES
Figure 1: Normal keratin formation…………………………………………………………..21
Figure 2a: Keratin coming up off of canal…………………………………………………...22
Figure 2b: Latent diabetes II case……………………………………………………………25
Figure 3: Summary description of mechanoreceptors of the EAC……………………….29
Figure 4: Relationship between keratin status and vagus reflex…………………………32
Figure 5: Ear-related red flags requiring medical referral…………………………………38
Figure 6: Typical timelines for sequela involved in dispensing tasks…………….………49
Figure 7: Frequency histogram showing the distribution of age .................................... 66
Figure 8: Monaural vs binaural fittings........................................................................... 68
Figure 9: Custom instruments vs post auricular (BTE) instruments .............................. 68
Figure 10: User Experience: Previous vs new users ..................................................... 68
Figure 11: Remake, FRC, and in-office modification rates ............................................ 69
Figure 12: Keratin status at each level of condition at initial evaluation......................... 71
Figure 13: Distribution of adaptation levels among participants .................................... 75
Figure 14: Histogram showing distribution of the levels of adaptation ........................... 76
Figure 15: Two cases of peeling EAC Keratin: Mechanical trauma, DMII case ............. 87
Figure 16: Two cases: Absent keratin, thick keratin ..................................................... 88
Figure 17: MedRx Video otoscope ................................................................................ 92
viii
LIST OF TABLES
Table 1: Medications known or suspected to contribute to EAC problems .................... 44
Table 2: Descriptive analysis of age of participants ...................................................... 65
Table 3: Descriptive analysis of participant fitting data .................................................. 72
Table 4: Descriptive analysis of remake, RFC, and in-office modifications ................... 71
Table 5: Descriptive analysis of keratin status .............................................................. 72
Table 6: Descriptive analysis of adaptation experiences ............................................... 76
1
CHAPTER ONE
INTRODUCTION
Overview
Despite unprecedented progress in hearing aid and assistive technology,
manufacturers and clinicians have continued to lose ground in penetrating an otherwise
rapidly growing hearing impaired market (Chartrand & Chartrand, 2004). To make
matters worse, the industry has continued to experience a return for credit (RFC) rate of
almost 20%, uncountable in-office shell and earmold modifications, and costly factory
remakes. Consequently, it has been entirely arguable that this state of affairs has been
producing a growing population of hearing impaired individuals who are convinced that
they cannot wear hearing aids. Many of these have found that they had difficulty
adapting to hearing aids, and complain of discomfort, non-acoustic occlusion,
uncontrollable variables in threshold sensitivity and other challenges that prevent them
from enjoying a favorable experience during the trial period.
Not only does failure to fit prevent a large segment of hearing impaired
individuals from participating more fully in an increasingly communication-intensive
society, it also increases the cost of every hearing aid that is ultimately purchased by
consumers (Barber, 2005). Concomitant with a failure to fit has been the common and
increasingly prevalent practice for consumers to use hydrogen peroxide, boric acid, and
other harsh solutions, including cotton swabs, in their routine personal ear care
(Infoscan data, 2003). Use of these products effectively removes and/or prevents
formation of the needed keratin layer (corneum stratum) of the external auditory canal
(EAC), causing loss of homeostasis and increased hypersensitivity of the external ear
2
neuroreflexes that can prevent comfort and adaptation to hearing aids and earmolds
(Chartrand, 2004).
Statement of Problem
In a recent pilot study, it was found that the degree of keratin (corneum stratum)
health in the EAC has a direct bearing on the frequency and degree of sensitivity of the
neuroreflexes of EAC, such as the Arnold’s or Vagus reflex (cough and gag), the
Trigeminal reflex (or “red reflex” of the tympanic membrane), and lymphatic reflex (or
swelling due to mechanoreceptor pressure). These reflexes were implicated in
preventing some hearing impaired individuals from adapting to hearing aids (Chartrand,
2005).
However, in everyday clinical practice, in all three sectors of the hearing
instrument dispensing community—hearing instrument specialists, audiologists, and
otolaryngologists—there has been a notable absence of recognition of the essential
neurophysiological tripwires that prevent many hearing aid consumers from successfully
adapting to wearing earmolds and hearing aids. If a correlation between keratin status
and adaptation success in wearing hearing aids could be established, then the next step
would be to recognize those chronic and acute conditions that contribute to a
deterioration or inability to produce and/or maintain a healthy layer of keratin in the
EAC. Consequently, it naturally follows that through an allied hearing health care team
approach involving each of these entities, as well as their manufacturing and
engineering counterparts, that more hearing aid consumers would enjoy higher rates of
success adapting and wearing hearing aids, especially those with the presenting
anomalies that otherwise prevent successful adaptation. This should also help the
3
hearing industry more deeply penetrate the hearing impaired market, which has for the
past decade or more become resistant to the industry’s ongoing efforts.
In approaching this study, there were at least two additional subsidiary questions
that will need to be thoroughly addressed. They are:
How might clinicians observe, identify, and counsel relative to potential
factors—personal ear care, clinical treatment, medication side-effects, chronic
disease, etc.—that may contribute to interrupting the natural processes that
develop and maintain keratin health in the EAC?
How might clinicians observe, identify, and counsel relative to effects of
hypersensitivity of the EAC neuroreflexes during hearing aid fitting and
adaptation process?
Therefore, the null hypothesis (Ho) has been stated as: External ear keratin
status has no correlation with one’s ability to adapt to wearing hearing aids. An
alternative hypothesis (Ha): External ear keratin status can be used as a predictor of
hearing aid fitting success.
Definition of Key Terms
Since many of the terms and constructs involved in this paper were not already in
the armamentarium of mainstream clinical practice, it was necessary to provide general
definitions of the key terms used throughout the study. Additionally, it must be kept in
mind that a refinement of these definitions, even the names of the terms themselves,
may change significantly as more research enables a greater understanding of these
concepts and terms. For instance, the term Trigeminal reflex was used here to denote
the widely recognized, but generally misunderstood Red Reflex, which is observed
4
during routine otoscopy. Though the trigeminal nerve in the epithelium of the EAC
simply acts in a mediating capacity, interacting with at least two other nerves at the
tympanic plexus (TP) of the tympanic membrane (TM) to produce the resulting vascular
and lymphatic stimulation, it is also the principal efferent (motor) player for causing
hypervascularization, and thereby a thickening of mass at the TM. For some hearing aid
users, this reflex does not cease or adapt after insertion of a typical hearing aid or
earmold. This can cause a disproportionate need for more hearing aid gain and/or
output in the in situ hearing aid prescription (Robert, Funnell & Laszlo, 2005; Chartrand,
2005; Smoliar, Smoliar & Belkin, 1999). Likewise, the Arnold’s branch reflex was
referred to here more broadly as the Vagus reflex, since the potential end results
(coughing, gagging, effortful phonation, nausea, myocardial tension) actually involve
several branches and interconnecting pathways of the Vagus (Chartrand, 2005; Amin &
Koufman, 2001; Hadjikoutis, Wiles, & Eccles, 1999). In the spirit of the foregoing, a list
of key terms and their brief definitions is presented alphabetically as follows:
Adaptation- The process by which a hearing aid/earmold is accepted by the
neurophysiological defense system of the EAC. Physiologically, usually requires
30-60 days; psychoacoustically, about 90-120 days.
Auditory Rehabilitation (AR)- Also known as Aural Rehabilitation, AR
encompasses the acquired ability to optimally utilize amplification, assistive
devices, and coping strategies.
Cough Reflex (CR)- Alternate term for the Vagus or Arnold’s reflex, elicited
upon insertion of any object into the EAC, especially an impression otoblock,
hearing aid, and/or earmold.
5
Earmold Modification- In-office modifications that are made to reshape,
resize, or change the physical configuration of a hearing aid or earmold in
response to visual observations or user reports of discomfort or other fitting
artifacts.
External Auditory Canal (EAC)- Generally refers to the pinna, concha,
aperture, and the external meatus inward to the tympanic membrane.
Keratin or Corneum Stratum- The outer layer of tissue of the EAC that grows
from the umbo of the TM outward toward the aperture of the EAC at the
approximate rate of 1mm/day.
Keratosis Obturans- The growth of extra layers of epithelial and/or corneum
stratum layers of tissue in the external auditory canal (Park, et al., 1999;
Chakeres, Kapila & LaMasters, 1985). In most advanced form (over several
years) it may envelope desquamated tissue, debris, and bacteria and appear to
be impacted cerumen (Naiberg, Berger & Hawke, 1984).
Lymphatic Reflex (LR)- Innervated by epithelial mechanoreceptors (hair
follicles, Meissner, and Pacinian corpuscles), presents varying degrees of tissue
swelling that are reciprocal to the degree of pressure upon insertion of hearing
aid or earmold. Like wearing a wristwatch, usually calms after a period of
adjustment (Nafe & Wagoner, 1941).
Maladaptation- The refusal of the EAC defense system to accept the hearing
aid/earmold, even upon following a prescribed wearing schedule, preventing the
user from wearing these devices comfortably and effectively.
6
Mechanoreceptors- Neurological receptors (i.e., hair follicles, Meissner and
Pacinian Corpuscles) imbedded in the epithelium of the EAC, which detect
movement, pressure, or perceived threat from a “foreign object”. These
receptors, in turn, send reflexive messages to various neuronal junctures along
and near the surface of the EAC and TM.
Non-acoustic Occlusion (NAO)- An own-voice perception of feeling plugged
when there is actually no acoustic basis for the sensation (i.e., Vagus reflex).
This is a purely tactile sensation caused when an earmold (vented or not) puts
sufficient tactile pressure upon the Arnold’s branch of the Vagus within the EAC.
Remake- Refers to a complete remake of the shell or earmold of a hearing aid
fitting at the factory. Ostensibly, remakes are usually done for the purpose of
alleviating discomfort, loose fit, acoustic feedback, or for cosmetic reasons.
Returns for Credit (RFC)- New hearing aids that are returned to the factory
because the user was unable to adapt or would not wear. While dispensers tend
to count only those returns for which a complete refund has been made,
manufacturers count every returned instrument as a credit return.
Stratum Corneum- The outermost layer of tissue lining the external ear canal,
known also as keratin. Growing outward from the region of the umbo of the
tympanic membrane at about 1mm per day, the stratum corneum keeps external
ears clean, maintains pH and hydration, and protects oversensitivity of the
neuroreflexes.
Trigeminal Reflex (TR)- Hypervascularization (blood and lymph fluid) and
physical swelling of the TM upon insertion of a otoscopic speculum, impression
7
otoblock, hearing aid, or earmold. In cases of hearing aid/earmold use, this may
artificially create the need for more gain and output in individuals that do not
adapt after a period of wear.
Tympanic Membrane (TM)- A three-layered membrane (epithelium, fibrous,
mucosa) located at the end of the EAC. It includes the physical landmarks of the
annular ring, tympanic plexus, pars flaccida, pars tensa, umbo and ossicular
connections.
Umbo- The cartilaginous structure that connects the ossicular chain to the
manubrium approximately center at the TM. In video otoscopy this becomes one
of the biomarkers for TM normality.
Vagus Reflex (VR)- A cough, gag, effortful phonation, nausea, etc. that may
be evoked in some individual upon insertion of an otoscopic speculum, ear
impression otoblock, hearing aid, or earmold into the EAC.
Wearing Schedule (WS)- A systematic plan adapted to a given hearing aid
user’s experience to help them adapt to wearing a hearing aid or earmold.
Involves psychosocial, psychological, psychoacoustic, dexterity, comfort, and
audibility considerations.
Brief Review of the Literature
A comprehensive review of the hearing health literature exposed a paucity of
scientific explanations relative to the role and importance of the keratin layer of the
EAC. Of equal concern was the lack of recognition in the literature of the neuroreflexes
that continue to complicate some consumers’ adaptation to amplification. What has
been studied in this regard has left more questions than answers.
8
For instance, Bloustine, Langston and Miller (1976) found that in a population of
688 adults in a clinical setting, the Arnold’s (Vagus) ear-cough reflex could be elicited
only in a mere 1.7% of subjects. Gupta, Verma and Vishwakarma (1986) found an
incidence of 4.2% in a similar clinical population of 500 adults. In both studies, a small
probe touching only a tiny area of the ear canal was used in an attempt to elicit a cough.
However, Chartrand (2005) found that an ear-cough reflex can be elicited in average of
37% of adults who wear hearing aids when an otoblock is inserted for purposes of
taking an ear impression, and the keratin layer was visibly absent. While there is no
practical equivalence for the stimulus used in the former studies, the latter study
represents what is experienced in daily hearing health practice everywhere. Other,
though rarer, subsidiary reflexes mediated by Arnold’s branch in the ear are also found
in the auriculo-palatal, auriculo-lacrimal, auriculo-cardiac and the ear-vomiting reflexes
(Gupta, Verma & Vishwakarma, 1986; Reid, 1922).
Moreover, just as important was the noted lack of audiological literature
recognizing the Trigeminal (Red) and Lymphatic (swelling) reflexes. Johnson and
Hawke (1981) presented one of the few expositions on these reflexes as they relate to
hearing health. Yet these reflexes are routinely observed during otoscopy (Trigeminal or
red reflex) and during fitting and post-fitting observations and modifications to help
overcome maladaptation to hearing aids and earmolds (Lymphatic reflex).
Consequently, specific representation of these reflexes by various designations in the
literature tended to be sparse and inconclusive (Robert, Funnell & Laszlo, 2005; Oliviera
et al., 2005; Chartrand, 2005; Hawke, 1981).
9
On the other hand, as we stepped outside the audiology literature into the
literature of neuroscience, physiological psychology, otolaryngology and dermatology,
we found a rich tapestry of studies, models, and theories from which to understand and
model the constructs and principles under-girding my hypothesis. For example,
Hadjikoutis, Wiles and Eccles (1999) described in intricate detail the reflex arc of the
Vagus in ear-cough that is elicited from motor neuron disease. Checkosky (1991)
presented temporal summation in the Pacinian channel relative to response to
vibrotactile stimuli. The epithelial distribution of Meissner’s corpuscles was explored by
Guclu, Bolanowski and Pawson (2003) and Pare et al. (2001), while Kryzanski (1997)
developed a model of membrane potential for mechanoreceptors in human skin.
Likewise, Nordin (1990) has performed microneurologic recordings to map the
innervation of the human face, representing much of the same afferent and synaptic
activity found in the human EAC, while Smoliar, Smoliar and Belkin, (1999) investigated
vascularization that is involved in the trigeminal reflex arc.
Pertaining to the role and importance of the keratin layer in the EAC, we explored
the otolaryngology literature, which examines pathologies arising from absent or
abnormal keratin development (Persaud et al., 2004; Entlink, 2001; Vrabic &
Underbrink, 2001; Naiberg, Berger & Hawke, 1984). Likewise, dermatology researchers,
such as Huntley (1995) and Prairie (2005) explored the effects of chronic conditions,
such as diabetes and Rosacea and their deleterious effects on production of
Keratinocytes in the human body.
Finally, in the audiology literature we found a widely held concern for the need to
resolve issues relative to failure to fit (Cox, Alexander & Beyer, 2003; Jenstad, Van
10
Tassell & Ewert, 2003; Pirzanski & Berge, 2002; Stakeholder Forum, 2001; Resnick,
1999). A significant problem existed in the lack of consensus about which issues were
most problematic. It is the purpose of this paper to answer the neurophysiological part
of that question, and in so doing provide the academic foundation necessary to supply
some of the missing pieces in the auditory rehabilitative puzzle (Wayner, 2005,
Chartrand, 2004).
Statement of Purpose
As noted above, the purpose of this study was to explore the relationship
between one’s keratin status and its possible effects upon the rate success of adapting
to hearing aids. This exploration applied to both new and experienced hearing aid
users, who have purchased new hearing aids during a specified time period. We
considered myriad health conditions, both chronic and acute, that may have a bearing
on keratin health and sensitivity issues of the mechanoreceptors that cause fitting
complications. It also involved pharmacological effects and personal care habits,
including that rendered routinely by medical professionals, in general, and hearing
health professionals, in particular, who have been contributing to the problem by their
routine advice.
This study also required exploration of ways to counsel, mitigate, treat, and refer
for such anomalies that potentially interfere with hearing aid adaptation. Hence, a team
approach was examined in terms of resolution of various physiological and
pharmacological challenges. Moreover, it was an important goal for this study to explore
the foregoing implications and how this new knowledge may foster positive changes in
tomorrow’s best practice standards throughout the hearing health field.
11
Research Expectations
During one pre-study trial of just 10 patient files, which were used to test various
measure sensitivities, we ultimately found a significant correlation (r = .886, p < .001)
between the visually ascertained status of the keratin layer of the EAC and the rate of
success in adapting to hearing aids. Although these patient files were chosen at
random, examining personnel had not yet been fully trained in interpreting the case
notes into solid, identifiable benchmarks that correspond with an established
measurement scale. Nor were there the types of control over confounds that was in
place for the actual study.
One confound that has yet to be fully addressed was how to rate the success
status of a fitting that was initially binaural but became a monaural fitting due to the
return of one hearing aid. If the reason for return was based upon economics rather
than failure to accept binaural amplification, the outcome might otherwise be safely
construed as successful adaptation. File reviewers were instructed to ferret out these
cases, and to eliminate them from inclusion.
On the other hand, where on the rating scale does the return of one hearing aid
due to adaptation issues fall? And, of course, a sample of ten subjects was much too
small to provide appropriate internal or external validity or reliability in the larger
universe of hearing aid users. So, the correlation in the new study, under the expected
rigor of the final study, was not expected to show as high because the earlier study
results (neuroreflex sensitivity) were taken at the start of the fitting process, whereas the
new study was showing relationships from a file review after the auditory rehabilitation
12
process has been completed. Even so, even a moderately positive correlation should be
enough to validate the concern over keratin status and hearing aid success.
13
CHAPTER TWO
REVIEW OF THE LITERATURE
Failure to Fit Deters Hearing Impaired Consumers
Throughout the literature, one finds almost unanimous consensus over the major
psychological and psychosocial deterrents for hearing impaired consumers’ failure to
seek help for their hearing and communication breakdowns. Consensus includes such
behaviors as lack of awareness, denial, vanity, social stigma and skewed cost/benefit
perceptions (Chartrand & Chartrand, 2004). Indeed, Ramsdell (1978) suggested some
years ago that the most significant problems presented by unmitigated hearing loss
were psychological, including depression and anxiety, defensiveness, distrust, and
social paranoia. Van Hecke (1994) noted the need for development of better
counseling methodologies to help hearing impaired individuals overcome negative
emotional responses to hearing loss, while Herbst and Humphrey (1980) long ago noted
the deleterious impact that hearing loss can have on quality of life for older adults who
forego obtaining appropriate help.
Regarding social stigma and vanity, the hearing aid industry has gone to great
lengths to overcome these reasons why reluctant consumers have not been seeking
help with hearing loss. Furthermore, cost/benefit issues have been addressed by
numerous researchers (Sweetow, Bratt, Miller & Henderson-Sabes, 2004 ; Peterson &
Bell, 2004; Crandall, Kricos & King, 1997). It is beyond the scope of this review to
provide an in depth exposition of these and other psychosocial barriers to hearing care.
However, these considerations are important for one to have a perspective of the
factors for which consensus may not have yet developed, such as the negative impact
14
on prospective hearing impaired consumers who put off purchasing hearing aids
because of someone else’s hearing aid experience (Kochkin, 1999). Indeed, it has been
reported that a sizable segment of would-be hearing aid users have been discouraged
precisely because of others’ negative experiences (Chartrand, 1999). Furthermore,
consumer websites are filled with alarm and caution about purchasing hearing aids, as if
doing so is fraught with trust issues not as prevalent in other areas of healthcare (Bell,
2006; Berke, 2006; Goddard, 2006). This irrational ambivalence can only contribute to
the psychosocial deterrents already keeping many hearing impaired individuals from
seeking help for their impairments. Ross (2005) sums up the effects of such perceptions
this way:
Everybody, it seems, has a friend or a relative who has had an
unsuccessful experience with hearing aids, and is quite vocal about it.
Many of these people, and there are too many of them, discourage
potential hearing aid candidates from trying one, since they "know" that
hearing aids are useless. Their attitude plays right into the usual
reluctance to accept amplification. (p. 3)
While some negative consumer attitudes appear to be changing (Bentler, 2000),
others linger on, because the hearing aid industry has yet to come to grips over
physiological factors that still prevent a sizeable portion of consumers from successfully
adapting to earmolds and hearing aid amplification. Hearing Industries Association
(HIA) annual reports still indicate credit return rates ranging from 18% to 21%, or about
the same rate as 20 or 30 years ago (Peterson & Bell, 2004; Ross, 2001; Kochkin,
1999). That translates into 18 to 21 of every 100 hearing aids manufactured are
15
returned and scrapped or refurbished for sale. Custom instruments comprise about 70%
of all instruments manufactured in the United States, while one hundred percent of
those that are returned for credit are virtually scrapped. Bray, Johns, and Ghent (2001)
report that this accounts for nearly 30% of the manufacturers’ initial cost of producing
the next new hearing instruments. These costs go far beyond the cost of wasted parts
and labor, but also in expenditures in customer service, billing, shipping, and costs
associated with losing future business. Hence, those who keep their custom hearing
aids are absorbing the cost of those that do not (Barber, 2005). In the case of over-theear (OTE) or behind-the-ear (BTE) instruments, the cost of returns is estimated at a loss
of about 15% of manufacturing cost, because most of these may be refurbished,
restocked, and resold back to the market, provided the actual time period of trials is
short, and wear and tear minimized (DigiCare, 2006).
Furthermore, studies published to-date show that newer technologies and
smaller-size instrumentation are burdened with a much higher return for credit rate than
the older, less costly technology. Part of the problem, of course, is consumer
expectations in cost-benefit factors (Peterson & Bell, 2004; Weighland et al., 2002).
However, it is arguable that a large part of the problem is that smaller instrumentation
encroaches upon physiological dynamics located deeper into the EAC than do larger
instruments. Sweetow et al, (2004) conducted a retrospective study in time-cost
analysis between three groups of hearing aid users: 1) Those that purchased and kept
their hearing aids, 2) those who exchanged their hearing aids for other models, and 3)
those who returned their hearing aids for a full refund. The time aspect for group 2 was
nearly twice as high as either of the other groups. However, group C brought about the
16
highest cost (or loss) to dispensers and manufacturers, particularly because a
disproportionate number of returned instruments fell into the higher tech category.
Hence, the higher the cost, the greater the expectation of benefits and an increased
propensity to return instruments for a refund. Weighland et al (2002) concurs with this
finding, noting that in 2001, HIA reported a return rate of 57% for some advanced digital
technology models versus only 16.7% for low/old technology devices. And these studies
do not even begin to address other major cost factors, such as factory shell/earmold
remakes and innumerable in-office shell and earmold modifications, many of which still
fail to satisfy complaints of discomfort and/or occlusion (Chartrand, 2006).
Recently, the hearing aid industry has responded to the persistent problem of
chronic returns for credit, remakes, and modifications—especially higher or more
frequent for digital and smaller instrumentation—by investing heavily into “niche
products”, such as costly implantable and retro-auricular hearing aids (Chartrand &
Chartrand, 2006). It comes as no surprise, for example, that a primary rationale for
implantable or bone-anchored hearing aids is that they bypass most complaints of EAC
discomfort and occlusion issues, but at several times the cost to the consumer and
reimbursement entities (Columbia University, 2006; Spyries, 2003). The newer OpenEar Over-The-Ear (OTE) and Receiver-In-The-Ear (RITE) instruments show promise for
those with mild losses, or normal hearing in the low frequencies to moderately severe
loss in high frequencies (Schweitzer & Jessee, 2006). However, most of these losses
will eventually progress out of range of these configurations, requiring utilization of
conventional earmolds (Rose, 2006). While these products fill an important need for
17
finite subsets of the market, they do not address the greater need of the larger market of
those using and/or needing hearing instrumentation (Chartrand & Chartrand, 2006).
Self-Assessment Studies and Reasons for Failure to Fit
In recent years, numerous self-assessment scales have been devised to gauge
levels of consumer satisfaction with hearing aids. The most commonly accepted scale
has been the Abbreviated Profile of Hearing Aid Benefit (APHAB), which is a shortened
version of the much lengthier Profile of Hearing Aid Benefit (PHAB). APHAB is a 24item self-assessment inventory on the functional before and after aspects of hearing aid
adaptation and benefits (Cox & Rivera, 1992). Of its four subscales--ease of
communication, reverberation, background noise, and aversiveness—not a single item
deals directly with physical discomfort complaints, sensations of occlusion, or own-voice
problems.
A more recent scale of consumer perceptions is the Satisfaction with
Amplification in Daily Life (SADL) scale. SADL is designed especially to measure
quality-of-life perceptions for experienced consumers who have purchased new hearing
aids (Cox & Alexander, 1999). Although SADL invites even more frank consumer
responses relative to problems with utilization of hearing aids, like its predecessor, it still
avoids even the allusion to the existence of discomfort, occlusion, or own-voice
complaints (SADL, 2006). However, these types of complaints head the list by
dispensers and audiologists as primary reasons for credit returns and remakes from
reports of patients (Chartrand, 2006; DigiCare, 2006). While consumer feedback on
functional issues (vis a vis APHAB and SADL) are immensely important to the industry,
18
it seems that of at least equal concern would be issues of comfort, occlusion, and ownvoice perceptions.
On the other hand, an analysis of the International Outcome Inventory for
Hearing Aids (IOI-HA), a seven-item survey comparing outcome data from various
research settings, reveals some inclusion of physical acclimatization issues (Cox,
Alexander & Beyer, 2003). Its co-authors note that aided outcomes negatively correlate
closely to mean average and standard deviations of patient subjective perceptions in
unaided state. In other words, the more difficulty consumers encounter in the unaided
state, the less objection they exhibit toward artifacts of acclimatization. Perhaps, by
these findings, consumers with greater perceived difficulties without hearing aids are
less bothered by physical fitting issues encountered with hearing aids.
Jenstad, Van Tassell and Ewert (2003) conducted a hearing aid-user study with
a post-fitting questionnaire covering five areas of complaints (gain, output, physical fit,
compression characteristics, and unwanted sounds), and found that patients were very
lucid in describing complaints relating to physical fit. Their scales of measure were
particularly instructive in drawing out these kinds of concerns. Indeed, their findings
agree with Resnick (1999), who reported from his study of consumer perspectives, that
“the literature tends to emphasize [only] the electroacoustic characteristics contributing
to improved performance” (p. 1) instead of testing out the validity and relevance of
current consumer satisfaction measures. In an effort to determine the relationship of
earmold fit and predicted real ear measures (and outcomes), Hoover and
Stelmachowicz (2000) report a positive correlation between a good fit and patient
perceptions of benefit (i.e., poor physical fit is often perceived as poor acoustic benefit).
19
Meanwhile, Humes and Wilson (2003) show in a longitudinal study of nine hearing aid
users that positive outcomes actually continue to improve even three years post-fitting,
albeit gradually and incrementally.
Finally, in testimony before the U.S. Food and Drug Administration (FDA)
Hearings Panel, in response to the Etymotic and Gudhear Petitions seeking approval of
over-the-counter sales of hearing aids, Magelin (2004) provides an excellent review of
the many physical complexities and variations that require keen professional skills to
help patients accommodate to amplification. Thus, physical acclimatization success
may be intricately intertwined with the skills and attention of a dispensing professional
working personally with each new hearing aid user, something that cannot be afforded
in a so-called over-the-counter practice setting.
Elasticity and Dynamicism of the Human External Ear Canal
It is entirely probable that the real variations between user reports of discomfort,
occlusion, and own-voice complaints are more physiological than psychological. In
other words, barring idiosyncrasies resulting from chronic illness, pharmacology,
personal ear care habits, and levels of professional care, there are likely more
similarities than differences between user experiences. These similarities are founded in
the predictable, yet variable elasticity and dynamic nature of the ear canal itself. Oliviera
et al. (2005) analyzed the ear canal volume changes that occur in 67 subjects and
found that 51% of subjects’ ears exhibit dimensional changes of more than 10% by
simply opening their mouths, while the remaining 49% showed significant changes up to
10%. In an earlier study by the same investigators they found a range of ear canal
changes of up to 20% in anterior displacement of the cartilaginous region of the external
20
canal accompanying such movements as chewing, smiling, and speaking (Oliviera,
Hammer & Stillman, 1992; Oliviera, et al, 1992).
When one considers that the majority of ear impressions are taken in fixed
mandible position, it is no wonder that problems of discomfort arise in so many hearing
aid fitting cases. Pirzanski and Berge (2002) present data gathered from a survey
conducted by 56 international doctoral audiology students participating in a distance
learning course on earmold technology. They estimated from that survey that more than
50% of impressions result in poor earmold fittings from professionals’ failure to
recognize the dynamic aspects of the human ear canal. It was further noted that only
43% of survey respondents utilized dynamic impression techniques. In other research,
failure to take dynamic ear impressions is a major contributor to failure to fit (Boys
Town, 2006; Stakeholder Forum, 2001). Indeed, Robert, Funnell, and Laszlo (2005)
identify a host of dynamic changes that occur on a perpetual continuum from the
aperture of the EAC all the way to the manubrium of the tympanic membrane. These
living changes conflict daily against inert earmold and hearing aid shell materials.
Natural, dynamic processes of the human ear canal tend to respond negatively to
the fixed, inert materials of hearing aids and earmold, and tend to treat hearing aids and
earmolds as invading foreign objects (Chartrand, 2003). This dynamicism excites the
mechanoreceptors imbedded within the tissues of the human epithelium each and every
time movement occurs. A case in point is the acclimatization process involved in
wearing a wristwatch (Nafe & Wagoner, 1941). Passage of time and relative cessation
of movement can cause neural firing to cease, in which case neural adaptation occurs
(Nafe & Wagoner, 1941). However, because mandibular and neck and facial
21
muscles—involved in smiling, chewing, talking, yawning, grimacing, etc.—never cease
movement, mechanoreceptors imbedded within the epithelium of the external ear canal
continue neural firing until another control mechanism is activated: The parasympathetic
process of a given reflex arc (Carlson, 2002). To subordinate and minimize the effects
that movement causes in the dynamic ear canal, therefore, requires dynamic ear
impressions that split the difference between dimensional extremes of change (anterior
to posterior and superior to inferior) so that the adaptation process can be shortened for
the hearing aid user (Chartrand, 2006). Hence, dynamic impression-taking alone can
help minimize adaptation to hearing aids as will be explained later in Chapter Five of
this paper. But there are other considerations for adaptation, most importantly—if our
hypothesis proves correct—the status and health of one’s layer of keratin that lines the
external ear canal.
The Role of Keratin in the External Ear Canal
In dispensing and audiological practice, perhaps the least known or appreciated
anatomical feature of the EAC is the stratum corneum or keratin layer of tissue. Yet the
health of the keratin layer determines the health of the human ear canal and negatively
or positively can affect the success of hearing aid adaptation (Chartrand, 2004). The
keratin layer is the outermost portion of the external ear canal (Johnson & Hawke,
1988). Its physiological role is critical in maintaining homeostasis and adapting
comfortably to hearing aids. Yet, though easily viewed through video otoscopy, its
status often goes unnoted during the normal course of dispensing activity (Chartrand,
2004).
22
Keratin is comprised of inorganic protein with no circulatory or neurological
system. Chemically, its structure is almost identical to that of human hair and nails
(Winter, Schweizer & Goerttler, 1983; Merck, 2006). In the EAC it completely covers
epithelial tissue, starting at a point near the umbo of the tympanic membrane and
traveling the entire length of the canal lumen to the aperture or opening of the ear canal
(Naiberg, Berger & Hawke, 1981). Not unlike the proverbial elephant in the living room,
keratin protein is arguably the most ignored part of the outer ear by hearing health
professionals overall. So important is its role in maintenance of the human ear canal,
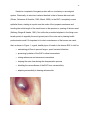
that, as shown in Figure 1, a good, healthy layer of keratin in the human EAC is vital for:
maintaining pH flora to prevent fungus, yeast, bacterial infections
preserving hydration of the EAC to allow homeostasis
mixing sebaceous and cerumenous secretions
keeping the ears clean during the desquamation process
shielding the neuroreflexes of the EAC from oversensitivity
adapting successfully to hearing aid earmolds
23
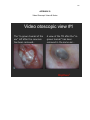
Figure 1. Normal keratin formation; desquamation lines spaced with appropriate
hydration.
When outwardly migrating keratin approaches the ear canal lumen, it terminates
just after contact with tiny hair follicles that grow inward, forming a kind of “ramp” that
lifts the desquamated (dead) skin cells, keratin and its cargo of debris from the
epithelium. In turn, minute accumulations of dead skin cells, debris and earwax are
steadily deposited into the concha of the ear for easy removal (Jahne & Cook, 1987).
Through otoscopy, keratin protein presents a “shiny” appearance as seen above. As
underlying new tissue grows steadily and haltingly outward from the umbo of the
tympanic membrane, its migration causes a “bunched up” appearance, forming circular
“lines” around the wall of the ear canal. In cases of dehydration or in response to some
medications these lines can be so close together as to appear granular (Chartrand,
2004: Johnson & Hawke, 1988).
Undisturbed, keratin is what shields the ear canal from bacteria, fungus, yeast,
amoeba and potentially septic debris. It also helps the epithelium or outer layer of skin
tissue—when coated with cerumenous and sebaceous secretions (which together form
“earwax”)—to maintain a slightly acidic pH environment of about 6.5. Hence, keratin is
the protective layer over the skin of the ear canal, without which the ear canal would be
more susceptible to invasion, injury and/or disease (Persaud et al, 2004; Slattery &
Saadat, 1997). Consequently, the absence of keratin in the ear canal may contribute to
many common complaints among hearing instrument users, such as:
chronic itching
hearing aid earmold discomfort
24
over-vascularization of the TM (via trigeminal and facial nerve pathways)
non-acoustic contact occlusion (via the Arnold’s branch of the vagus)
predisposition for chronic externa otitis (fungal, bacterial, etc.)
oversensitivity of the neuroreflexes of the EAC
The natural desquamation of tissue in the ear canal is such that tissue grows
outward from near the umbo (or center point of the eardrum) to the aperture of the ear
canal (Robert, Funnell & Laszlo, 2005). This natural process generally requires about
three months to migrate the full length of the canal at the rate of about 1mm per day.
So, if one were to place a piece of sand on the tympanic eardrum today, about three
months from now that person could remove the same piece of sand from the bowl or
concha of the ear by fingertip (Chartrand, 2004). Left undisturbed, then, healthy ear
canals are self-cleaning and wax impaction is rare (Jahne & Cook, 1997). Abnormally
low pH (below 6.5) often leaves the ear canal dry with a host of extant skin problems,
such as psoriasis, eczema, chronic external otitis, contact dermatitis, allergy and
abnormal cell growth such as basal and squamous cell carcinomas. After removal via
cotton swab trauma or scratching with any foreign object it requires about 10—14 days
for a good, strong layer of keratin to re-form. Hence, frequent use of cotton swabs will
effectively negate keratin formation (Chartrand, 2004).
In addition, frequent use of ear preparations containing boric acid or hydrogen
peroxide solutions not only destroy keratin and layers of epithelium, they also eliminate
the water repellent ability of same and leave the external ear canal at risk for chronic
otitis externa. Furthermore, these acids can inhibit cerumen formation, as well as
interrupt natural desquamation of tissue and the regrowth of the badly needed keratin.
25
Unfortunately, such harsh solutions are the mainstay of today’s over-the-counter
otopharmacopia. It must also be noted that an infected ear or one in which immunology
has been comprised will generally not exhibit keratin (Winter, Schweizer & Goerttler,
1983), just such an ear cannot normally secret cerumen and sebaceous secretions into
earwax (Jahne & Cook, 1997). So, an external ear without visible keratin (via video
otoscopy) may be considered an abnormal external ear (Chartrand, 2006).
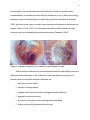
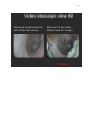
Figure 2: a) Keratin coming up off of canal; b) Latent diabetes II case.
Some common consumer and professional practices that inadvertently remove or
disturb the vital keratin layer of the external ear canal and which can set up one’s
external canal for the above-described problems are:
daily use of cotton swabs
insertion of foreign objects
frequent use of boric acid and/or hydrogen peroxide solutions
aggressive cerumen removal
dry and/or overly tight oto-block during impression taking
forcing one-size-fits-all earmolds into the ear
26
In cases where keratin has been removed due to any of the above described
methods or has not formed normally, especially in cases of abnormal cellular pH of the
body extant (e.g., via chronic dehydration, diabetes mellitus II, gout or candida), a host
of irritating, potentially dangerous organisms such as fungi (aspergillus favus, etc.),
yeasts (candida parapsilosis), pseudomonas aeruginosa (gram positive) and
streptococcus areus has been found growing in the ear canal and on the earmolds worn
by hearing aid users (Kemp & Bankaitis, 2000; Jahne & Cook, 1997). However, once
the offending practices cease, later video otoscopy should reveal an ear returning to
normal homeostasis and health. Likewise, unless there is underlying pathology, patient
complaints usually resolve on their own. If not, medical referral is indicated.
As one will see in Figure 2a, mechanical cotton swab trauma can cause early
separation of the keratin from the underlying epithelium. A differentiating factor, in this
case, is the visible new growth of keratin in front of the disturbed keratin, indicating that
the homeostasis of the ear canal has otherwise remained relatively normal long enough
for new keratin to form beneath. However, in cases of chronically abnormal pH
conditions, such as in rapidly developing diabetes mellitus type 2, keratin often “peels
off” with a snake-skin appearance during early separation from underlying epithelium. In
this particular case, there are no signs of new keratin coming from beneath the shed
keratin layer. Figure 2b (above) shows a typical case of undisclosed/untreated or latent
diabetes mellitus II and hyper/hypoglycemia, a common pre-diabetic condition
(Chartrand, 2003; Chartrand, 2004).
Mechanoreceptors of the External Auditory Canal
27
Mechanoreceptors are the underlying enablers of dynamic sensory and motor
function in EAC immunology. They incorporate the ability to sense when any object,
bacterium, and temperature and/or barometric change that enters or occurs in the EAC.
For instance, lymphocytes, leukocytes, cytokines---both pro- and anti-inflammatory, and
an array of immunological warriors, such as T-helper cells, T-cells, macrophage cells,
and immunoglobulins (IgE, IgG, etc.) receive significant excitatory and inhibitory
information based upon neurotransmissions arising from mechanoreceptors that cover
every square millimeter of epithelium in the EAC (Kryzanski, 1997).
Nordin (1990) developed a series of microneurographic recordings
demonstrating the densities and afferent-efferent (sensory-motor) roles of myelinated
and unmyelinated axons and interneural connections for facial, infraorbital, and
supraorbital mechanoreceptors, such as Pacinian corpuscles, Meissner Corpuscles,
and hair follicles, over the area of the human face. Their model is particularly useful in
demonstrating the intricate sensory and motor aspects of these mechanoreceptors in
terms of interaction with the autonomic nervous system. Watanabe (2004)
demonstrated the immunological role and cytoplasm formation of cells of Schwann and
Merkel by mechanoreceptors imbedded in the mucosal layer of the middle ear, which
also constitute the most inside layer of the TM. These mechanoreceptors have been
found to be involved in the production of IgE immunoglobulins in allergy response. Pare
et al. (2001) further examined Meissner corpuscle’s and found low threshold sensitivity
for detecting immunochemical properties associated with nociception, or detection of
physiological inflammation and/or pain.
28
Furthermore, Van Boven (2000), in an experiment to determine the spatial
resolution associated with the deeper situated Pacinian corpuscles found that no such
sensitivity existed at this depth in terms of fine sensory input. However, Pacinian
corpuscles still played a more important role in detecting deep movement and pressure,
such as the kind found in tight fitting earmolds. Moreover, each mechanoreceptor
involves both sympathetic and parasympathetic fibers, enabling acclimatization over
time. In other words, neural firing caused by given stimuli—such as adapting to an
earmold in the ear, a watch on one’s wrist, or a pair of new shoes on one’s feet—that
occurs over a period of gradual acclimatization will eventually cease firing (Nafe and
Wagoner, 1941). See Figure 3 for a summary description of the mechanoreceptors of
the EAC. The role of each mechanoreceptor and how they might interact with the
adaptation process of hearing aids are as follows:
Hair follicles reside in approximately the outer half of the EAC. They detect
fine air and mechanical movement and send neurochemical signals to the
tympanic plexus of the tympanic membrane and other areas of the external
ear, some of which terminate with other mechanoreceptors. Hair follicles, along
with Meissner corpuscles, appear to be directly involved in inciting the “red
reflex”, which can be defined as the hypervascularization reflex at the superior
portion of the canal lumen and is medial to the superior quadrants of the
tympanic membrane. This may be easily demonstrated with insertion of the
speculum during routine video otoscopy, manifesting with progressive dilation
of the vascular system of the EAC. Unlike the other mechanoreceptors, hair
29
follicles appear to stop firing quickly almost immediately after movement stops
(Nafe & Wagoner, 1941).
EAC Mechanoreceptors
Hair follicles
Senses slight air movement, incites
vascular activity at TM
Meissner’s Corpuscles
Senses light pressure near surface of
epithelium, sends signal to tympanic
plexus (Note: In complete reflex arc ceases
firing upon cessation of movement)
Pacinian Corpuscles
Senses deep pressure in mid-level of
tissue, sends signal to tympanic
plexus region (Note: Excites cytokine and
lymphocyte production)
Vagal stimulation (via
Arnold’s Branch)
Trigeminal (Efferent neurons)
/Facial (Afferent neurons)
Evokes various reflexes, including
gag, cough, cardiac constriction,
nausea in stomach
Controls vascularization &
lymphatic activity (Note: Some aspects
have no parasympathetic response)
Figure 3. Summary description of mechanoreceptors of the EAC and their
characteristics.
Meissner corpuscles are located near the surface of EAC epithelial tissue,
directly under the keratin or stratum corneum layer. When keratin is absent,
Meissner corpuscles are particularly active, causing a sensation of movement,
itching or other aberrant activity in the EAC. These mechanoreceptors detect
movement, light pressure, temperature and barometric changes, and likewise
30
send and receive messages from various points in the neurological system,
including the tympanic membrane, the limbic-modulated vascular system, and
cranial and cervical nerves. Nociceptor motor excitement involves inflammation
and pain reactions to trauma, and can be extremely involved with acute tissue
swelling during otitis externa. Though fast acting, Meissner corpuscles slowly
stop firing when movement or changes cease (Nafe & Wagoner, 1941).
Pacinian corpuscles are found deeper within the epithelial tissues of the EAC,
and also become more active when keratin is absent. Pacinian corpuscles
primarily sense pressure, inciting leukocyte activity in the affected areas of the
ear canal. They are particularly implicated in stimulating Arnold’s branch of the
Vagus, and in inciting the EAC reflexes of cough, gag, nausea, and other vagal
responses to invasion of the EAC. In addition, Pacinian corpuscles are implicated
in (nonpathological) chronic upper respiratory congestion or irritation in cases of
impacted cerumen (Chartrand, 2004; Jegoux, Legent, Beauvillain & de
Montreuuil, 2002). In addition, Pacinian corpuscle neural firing ceases long after
cessation of movement or pressure, but over time begins to adapt to prosthesis
via parasympathetic activity (Chartrand, 2006; Nafe & Wagoner, 1941).
Vagus (Arnold’s) reflex involvement in the External Auditory Canal. Of all the
neuroreflexes implicated in cases of hearing aid rejection, the Arnold’s branch of Vagus
appears to be at the top of the list, causing complaints of non-acoustic occlusion,
sensations of fullness, and varying manifestations of the reflexes of cough, gag,
nausea, and/or effortful phonation where pressure is applied in the ear canal (Amin &
Koufman, 2001; Birsch, Logeman, Rademaker, Kahrilas & Lazarus, 1994). Chartrand
31
(2005) found in a study of 27 hearing aid users, that 37% exhibited a marked cough/gag
reflex during otoblock insertion before an impression was made. During hearing aid
wear in these same patients, a lesser number exhibited non-acoustic occlusion where
increased earmold venting made little difference. These and other reflexes have already
been noted in the literature in a tiny subset of the general population (Gupta, Verma &
Vishwakarma, 1986; Bloustine, Langston & Miller, 1976; Reid, 1922), while recent data
shows a much higher prevalence (Chartrand and Chartrand, 2006).
A differentiating factor that tends toward determination of Vagus sensitivity in the
EAC is the thickness or presence of keratin. Chartrand (2005) noted that male EAC
keratin, on average, is about 30-40% thicker than female EAC keratin; but that the
relative sensitivity differences were about the same between the thicker keratin layer of
male ears compared to the thinner keratin layer of female ears. However, when the
keratin appeared thin in either case, sensitivity would increase almost exponentially.
Figure 4 shows the correlation (r = .886, p < .001) between the keratin status of 27
hearing aid users for both sexes—in this case, 18 males, 9 females; aged 47-89 with a
mean age of 68.3 years—displayed on a scatterplot graph (Chartrand, 2005).
32
3.5
3.0
2.5
2.0
1.5
1.0
.5
0.0
-.5
-.5
0.0
.5
1.0
1.5
2.0
2.5
3.0
3.5
KERATIN ST ATUS
Figure 4. Relationship between keratin status and vagus reflex in the ear canal upon
otoblock insertion.
A popular ear care trend for participants exhibiting the most sensitive Vagus
reflex was the inadvertent removal of keratin by frequent use of cotton swabs. Other
contributing factors were the self-treatment of itching by using hydrogen peroxide, boric
acid, or other caustic over-the-counter solutions. Yet others exhibited systemic
pathologies, such as acidosis, diabetes mellitus type 2, or gout. In each case, keratin
was either absent or was disturbed, causing greater sensitivity of the Vagus and other
neural reflexes. It is also important to note here that when participants refrained from
using cotton swabs or offending solutions in their EACs that, in most cases, visible
keratin began forming again after about two weeks’ time. In all cases where a gentle
botanical solution was used, such as Miracelltm Botanical Solution, keratin status not
only improved, but Vagus and other sensitivities subsided (Chartrand, 2005; Chartrand,
2002). One factor separating the cited study from the current one is that these
sensitivities and complaints were evoked at the start of the dispensing process, not from
33
a later file review as in the new study. Hence, as stated earlier, correlation between
keratin status and neuroreflex sensitivities should show much higher on the earlier study
than in the new study, which is an historical file review after all rehabilitative
considerations have been addressed.
Trigeminal (Red) reflex and its effect during otoscopy, impression taking, and
when wearing hearing aids. Adam Politzer (1835-1920) is credited as being the first
otolaryngologist/physician-researcher to trace the synaptic connections throughout the
Trigeminal nerve branch that innervate the tensor tympani muscle of the human middle
ear (Entlink, 2001). In doing so, Politzer helped discover the intricate autonomic
relationship between the Trigeminal nerve (Cranial Nerve V) and the external and
middle ear region, both from the standpoint of function and immunology. Functionally,
Trigeminal innervation of the tensor tympani muscle helps maintain air pressure
equalization between the outer and middle ear sectors. Even in most cases of
Eustachian tube dysfunction resulting from inhalant allergy or mild otitis media—when
the Eustachian tube fails to allow pressure equalization, drainage, and protection—the
voluntary or involuntary act of a yawn or a swallow is usually enough to send the tensor
tympani muscle into spasm (sensed as a flutter on the TM) that is strong enough to
force open the isthmus of the Eustachian tube located just below the hypotympanum
(DiMartino, Walther & Westhofen, 2005).
Another important function of Trigeminal innervation of the EAC and middle ear
region is stimulation of blood and lymph fluid vascularization. Smoliar, Smoliar, and
Belkin (1999), in an experimental study, traced the rich intraneural network of the
arterioles and venules of the trigeminal ganglion capsule or “neural plexuses”. The
34
tympanic plexus region of the TM, for instance, is innervated by light tactile movements
or temperature changes as far distant from the TM as the aperture (or entrance) of the
EAC. In that region, hair follicles and Meissner corpuscles detect stimuli as part of a
defense mechanism to warn the delicate tympanic membrane of pending attack of a
foreign object, insect, or other offending intruder. As mechanical movement continues,
the Trigeminal branch (V3) at the tympanic plexus of the TM receives interneural stimuli
from the tympanic plexus branch of the Facial nerve (VII), which is distributed along the
surface of the epithelium of the canal lumen. There, afferent sensory fibers of the Facial
nerve fibers receive synaptic firing from both mechanoreceptor hair follicles and
Meissner corpuscles near the surface of the epithelium, and deeper still from Pacinian
corpuscles.
Revisiting an earlier described example, insertion of an otoscope speculum can
set off this chain of events with increasing intensity, causing rapid dilation of
surrounding blood vessels just prior to and at the TM. In cases of hypersensitivity, this
rapid and pervasive dilation may appear through otoscopy as a mild yet acute otitis
media, although no pathology is actually occurring (Hawke, 1981). Although
manifestations of this reflexive action vary significantly from individual to individual, its
presentation in nearly all cases may be considered normal and not representative of
any pathology. This phenomenon has been called the “red reflex” of the TM during
otoscopy (Chartrand, 2006).
A concomitant or complementary action occurring with the red reflex, but from
deeper tactile pressure is a less rapid dilation of the lymphatic system by the same
interneural mechanisms. The lymphatic vasculature involved in the red reflex was
35
discovered many years ago by Reid (1922) when he traced the lympathic system of the
head and neck region of the human body. Reid and colleagues found between 10 and
15 lymph nodes involved with the tympanum (middle ear), hypotympanum (adjacent the
inferior connection of the tensor tympani to the Eustachian tube), and in the entire
region surrounding the external meatus (EAC). They were able to trace the
immunological reflex arc arising out of the parotid gland for defending the region from
infection, excessive pressure, and mechanical or other trauma. Their work is
foundational to our understanding of the immunological defenses of the external and
middle ear region today.
Pertinent to this review is the role an oversensitive Trigeminal or red reflex (which
includes lymphatic activity) plays on potential complication of user perception of hearing
aid amplification. In the Chartrand (2005) study of the neuroreflexes, it was found that
individuals who displayed significant vascularization during otoscopy also tended to
experience a “damping effect” at the TM when wearing hearing aids. It was
hypothesized, but not tested in the study, that in individuals who require more in situ
gain and output with their hearing aids than predicted may be lacking the
parasympathetic function in this reflex arc. Therefore, they are often referred to by
dispensing professionals as “power junkies”, indicating that as they wear their hearing
aids over time (usually after about 20-40 minutes), their need for gain beyond predicted
(target) gain mysteriously increases. It has been observed by many that these cases too
often result in excessive remakes, circuit changes, and returns for credit (Chartrand &
Chartrand, 2006).
36
Lymphatic (swelling) reflex. As noted earlier, the mechanisms of the lymphatic
reflex of the external meatus appear to be first discovered by Reid and colleagues as
they traced the lymphatic branches arising out of the parotid gland (Reid, 1922).
However, unlike the light tactile sensitivity pressure exciting the red reflex, the lymphatic
reflex appears almost entirely innervated by innervation of the Pacinian corpuscles
residing deep into the epithelium. It has been observed in private practice that in some
cases where pseudomonas, bacterial, or aspergillus infections, that aggressive use of
cotton swabs can cause such a violent lymphatic reflex action as to close up the entire
ear canal lumen, thus making insertion of a hearing aid or earmold uncomfortable if not
impossible (Chartrand, 2003a). In many cases, the offending infection lies dormant until
disturbance via cotton swab trauma. Schoppmann (2005), in explaining the role and
function of lymphatic reflexes in general, notes that:
The lymphatic vascular system is necessary for the return of extravasated
interstitial fluid and macromolecules to the blood circulation, for immune
defense and for the uptake of dietary fats. Impaired functioning of
lymphatic vessels results in lymphedema (p. 4503).
Because of the interconnectedness and pervasive action of the lymphatic reflex
and the system that supports it (Stewart, Quick, Zawieja, Cox, Allen & Laine, 2006;
Johnson & Hawke, 1981), it is no wonder that this is often the tumor staging mechanism
in cases of malignant carcinomas (Smoliar, Smoliar & Belkin, 1999). Schoppmann
(2005) further explores these mechanisms, demonstrating how infection that
overwhelms the parotid gland can spread to other lymph nodes and areas of the ear
anatomy. Likewise, these are the same routes and mechanism that enable metastasis
37
of carcinogenic cells to spread throughout the human body (Tille & Pepper, 2004;
Takahashi, Yoshimoto & Kubo, 2004; Van de Gaag, 1986).
The lymphatic reflex appears to be particularly active in the bony isthmus
region of the EAC, providing a possible explanation of why completely-in-the-canal
(CIC) fittings have experienced such inordinate rates of returns for credit, remakes, and
in-office modifications (Chartrand, 2006). For the outer portion of the external canal is
supported by cartilage emanating laterally from the auricle, extending about ¾” to 1” into
the ear canal; medially and toward the TM. However at the isthmus there is no
cartilaginous support, but instead a rigid, immovable temporal bone, where theoretically
CIC instruments are expected to produce an acoustic seal. It is in this region where the
epithelium thins from seven thick layers of skin tissue to three thin layers, and where
lymphatic response is most active, with or without intact keratin (Staab, 1997). The
problem of adaptation and reports of discomfort are dramatically increased when
hearing aids are worn in this region of the EAC (Chartrand, 2006; Oliviera, 1997).
Effects of Medically Treatable Conditions on Physical Adaptation to Hearing Aids
Potentially complicating all of the above constructs are myriad acute and chronic
health conditions, many of which are medically treatable, and therefore referable.
Included in the 1977 FDA Hearing Aid Rule are many of the physical conditions that
may be observed during the hearing aid evaluation via video otoscopy, patient report,
case history, or by a combination of all three (Chartrand, 2003b). The eight Red Flag
conditions required of hearing instrument specialists and audiologists to check for and, if
existing, make timely referral to a licensed physician, preferably one trained in diseases
of the ear, are shown in Figure 5 (below).
38
Observation & Referral: The FDA 8 Red Flags
I. Visible congenital or traumatic
deformity of the Ear
II. History of active drainage
V. Unilateral hearing loss of sudden or
recent onset within the previous 90
days.
VI. Audiometric air-bone gap equal to
or greater than 15dB at .5KHz, 1KHz, &
2KHz.
III. History of sudden or rapidly
progressive hearing within the
previous 90 days?
VII. Visible evidence of significant
cerumen accumulation/foreign body in
the ear canal.
IV. Acute or chronic dizziness
VIII. Pain or discomfort in the ear.
Figure 5. Ear-related red flags requiring medical referral (Federal Register, 1977).
The above “red flags” represent clinical conditions or symptoms that have
evolved into universally verifiable indicators, for which optimal treatment potentially
involves the guidance of a medical doctor. However, subclinical conditions, which may
or may not require medical diagnosis and treatment according to the judgment of the
attending professional, may involve: (1) Chronically itching ears, (2) superficial capillary
bleeding of the external ear canal, (3) Eustachian tube dysfunction due to inhalant
allergy, and (4) other abnormal presentations or complaints (Chartrand, 1999). Of
course, it is beyond the scope of this paper to cover these is substantial detail.
However, they need to be considered within the larger constellation of medically
treatable conditions that may serve as barriers or complications in adapting to hearing
instrumentation. Other conditions, not listed among red flag conditions, but which may
adversely impact successful adaptation to hearing aids and earmold are:
39
Diabetes Mellitus- Diabetes mellitus I (DMI) and diabetes mellitus II (DMII)
head the list of chronic conditions encountered in hearing healthcare because of
their prevalence within the population that is most often evaluated for hearing
loss (Chartrand, 2003c). However, there are significant differences between
these two manifestations in terms of both numbers and how they present hearing
health complications. For instance, DM I shares almost none of the concomitant
symptomology that comprise the DMII condition, such as hypertension, obesity,
hyperlipidemia, and inflammatory disease (Sandeep & Hayward, 2003). Also,
the vast majority of the population for DM II is primarily older adults, the primary
market for hearing aids. Even in the general population, there are many times
more DMII than DMI cases in the United States (Mokdad, Ford & Bowman,
2000). Hearing health professionals should be particularly cognizant of the
differences between these two populations and also their presenting symptoms
for file notations, counseling considerations, and possible referral for medical
diagnosis and treatment. Huntley (1995) explored systemic and metabolic
changes that occur in cases of DMII, many of which affect epithelial sensitivity,
inability to produce EAC keratin, and neurological artifacts affecting audition.
Chartrand (2003c) presented a comprehensive review of the potential effects that
active DMII can have on hearing aid fittings and considerations for resolving
problematic artifacts. As a result, considerations for cases of DMII may involve
special earmold materials and acoustic designs, strategic loudness growth and
loudness discomfort accommodations, allergic response, central auditory
neuropathy, and chronic infections and irritation presenting discomfort and
40
maladaptation to hearing aids. Moreover, responding to many of the foregoing
considerations may require communication and interaction with other health
professionals as appropriate.
Immunocompromised individuals- Conditions such as HIV, Hepatitis,
Chronic lung disease, Herpes Zoster, Rheumatic Fever, and Diabetes (as
described above), may prevent normal homeostasis in the EAC. For instance,
seriously elevated levels of blood sugar (as in DMII and during acute infections)
can prevent the formation, differentiation, and/or migration of keratin (Prairie,
2005; Vrabic and Underbrink, 2001; Steuer, Hofstadter, Beuth, and Strutz, 1995).
Antibiotic resistance and Candida overgrowth- Well-known today is a
phenomenal rise in antibiotic resistance in the general population (Barnett and
Klein, 1995; Samonis, Gikas & Anaissie, 1993). It really doesn’t matter for which
purpose the antibiotic is prescribed, organisms develop in the human body over
time rendering the antibiotic less and less effective over time. But a greater
concern for our purpose here with the proliferation of antibiotic prescription is the
growing trend in chronic and acute Candida albicans overgrowth in the
population, including chronic cases in the EAC region, which cause hearing aid
discomfort and maladaptation (Bento, 2006; Great Plains, 2006; Barnett & Klein,
1995; Kinsman & Pitblado, 1989; Kennedy & Volz, 1983; Ostfeld, Rubenstein,
Gazit & Smetana, 1977). The foregoing citations represent a continuum of
research discoveries that advise against routine antibiotic therapy for many
chronic ear conditions (Sahelian, 2006). The spectra of fungi, protozoa, and
amoeba (pseudomonas) are particularly troubling during Candida overgrowth
41
(Handzel & Halperin, 2003; Vrabic & Underbrink, 2001; Barnett & Klein, 1995;
Huntley, 1995). In such cases, it is best not to prescribe antibiotic suspension for
otitis externa, but instead anti-fungal (i.e., Burrow’s solution, 5% aluminum
acetate, selenium sulfate, and tinactin OTC solution) or anti-protozoa (i.e.,
Ciproflaxin Otic Solution HCL) solutions to reduce antibiotic resistant organisms.
Some cases may require oral antifungals, such as Ketoconazole, while in such
cases, systemic Ciproflaxin may be the safer choice (Chartrand, 2006). These
are mentioned here, because of their relative rarity in general practice in the
treatment of chronic external otitis which usually only presents when hearing aids
or earmolds are worn (Chartrand, 2004).
Benign and Malignant growths in the EAC- Because of metastasis via the
lymphatic system, cancerous cells may proliferate anywhere in the human body.
An irritated ear, or one that is immunocompromised, is particularly vulnerable to
opportunistic organisms. Handzel and Halperin (2003) explore necrotizing
malignant external otitis and the pathways that can result in more persistent
forms of breakdown in the underlying EAC structure. Dash & Kimmelman (1988)
explain the otopathological cycle caused by chronic tissue disturbance, and oversecretion of pro-inflammatory cytokines and chemokines that result in pain,
swelling, and inflammation in head and neck regions. This immunocompromised
state may further lead to syringomas (or ceruminomas) in the EAC (Janhke,
1976), actinic keratosis (Skin Cancer Foundation, 2006), or other forms of
carcinomas, such as basal cell or squamous cell in the EAC (Chartrand, 2006).
Schot et al. (1992) conducted a study of thirty patients with one-sided parotid
42
neoplasm that were treated with radiopathy and surgery. They found that while
surgical treatment seemed to have no detectable effect upon hearing levels the
radiological exposure caused significant dose-effected hearing loss in the high
frequencies. All of these may develop undetected in the course of hearing aid
fittings, only to complicate and frustrate adaptation success.
Potential Medication Side-Effects Impacting the EAC
More than 180 prescription medications have been found to adversely affect
the human cochlea (Edmunds, 2006; Briggs & Gadre, 2001). Indeed, these suspect
medications garner the lion’s share of research attention, while medications affecting
the human external auditory canal are only peripherally explored (Kavanaugh, 2007),
while much is done for veterinary external ear issues (Haar, 2003). It is not our
purpose to include a review of vestibular or cochlear ototoxicity, but instead to focus
upon external ear effects.
Table 1 lists medication classifications that have been found to adversely
affect the human EAC, directly or indirectly. Although most of these medications are
generally not prescribed for ear-related conditions, they exhibit varying levels of effects
upon hydration, immunological, and dermatitis issues. The effects of these otherwise
needed medications on the human EAC need to be considered when they appear to be
causing cases of failure to fit. Moreover, probably the most universal effect of all nearly
all of them is that they cause or contribute to dehydration. Some stimulate production
and secretion of antidiuretic hormone (ADH) production, which can significantly affect
electrolyte and cellular osmotic activity (Chartrand, 2007). Others interrupt
homeostasis, delay healing, and interact adversely with other substances, including
43
food and other drugs. Any medication that exacerbates chronic semi-dehydration
causes a loss of keratin formation and sets the stage for chronic otitis externa problems
(Chartrand, 2003b). These facts point to a need to keep in mind that pharmaceutical
solutions were meant to be temporary, rarely permanent, solutions to healthcare issues
until underlying causes can be addressed. To not follow that paradigm of healthcare
risks setting up far too many hearing impaired individuals with limited auditory
rehabilitative options, especially hearing aids and cochlear implants.
44
Table 1
Medications Known or Suspected to Contribute to EAC Problems
Medication
Classification
Analgesics
Antianginals
Antibiotics long-term
Antidepressants
Antigout drugs
Antihistimines
Antihypertensives
Antilipemics
Antineoplastic agents
Antipsychotics
Bronchodilators
Cholinergics
Corticosteroids
Diuretics
Estrogens/Progestins
Fluoroquinolodes
Hematologic Drugs
Immunosuppresants
Laxatives
N.A.I.D.s
Propylene Glycol
(Mixing agent)
Sedative-Hypnotics
Spasmolytics
Sulfonamides
Thrombolytic enzymes
Drying
Effects
X
X
X
XX
XX
XX
XX
X
X
XX
XXX
X
XX
X
X
X
XX
XXX
X
XX
X
X
X
Inhibits
Epithelium
X
X
Inhibits
Keratin
Fungal
Growth
Contact
Dermatitis
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
XXX
X
X
X
X
X
X
X
X
XX
XX
XX
X
X
45
CHAPTER THREE
METHODOLOGY
Restatement of the Problem
The significance of this research question was grounded in the fact that one of
the most perplexing challenges in the hearing aid industry today is the tremendous loss
caused by unnecessary shell/earmold modifications, remakes, and returns for credit
(Ross, 2002; Mims-Voll and Jones, 1998). For consumers that often meant delay and
less-than-optimal experience with hearing aids. Furthermore, the current state of affairs
meant that many would-be hearing aid users are discouraged from even trying hearing
aids due to others’ reported experiences, thus depriving them of the auditory,
communicative, and cognitive benefits of amplification entirely (Chartrand & Chartrand,
2004). Granted, not all of these problems were the result of physiological maladaptation.
Other prominent causes for failure to fit involved acoustic and psychoacoustic
limitations, programming and technological challenges, and compliance or perceptive
shortcomings on the part of hearing aid consumers (Kochkin, 2000).
However, there has long been a notable lack of research in the hearing aid field
in the underlying neurophysiological principles that can dictate outcomes for a good
many attempts to adapt to amplification. This disregard—despite substantive progress
in the technologies that can effectively aid the defective human auditory system—was
further evidenced by:
A marked lack of progress in reducing remakes and RFCs throughout the
industry (Peterson & Bell, 2004). Recent advancements in laser-assisted
earmold technology have made only minimal improvements in the rates of
46
remakes, returns for credit, and in-office modifications (Stakeholder Forum,
2001).
Earmold technology still generally subscribes to the non-dynamic model of the
human ear, evidenced in ineffective impression-taking methodology, otoplastics
that are chemically active in the ear, and in the general lack of acknowledgment
of real physical adaptation processes (Chartrand, 2006; Kolpe and Oliviera,
2003; Stakeholder Forum, 2001; Grenness, 1999).
Near total disregard of the external ear’s defense mechanisms, particularly
those designed to reject invading bacteria and “foreign objects”, which as far as
the ear is concerned includes hearing aids and earmolds. Mechanoreceptors and
neuroreflexes, including their sympathetic and parasympathetic roles, remain
among the least considered underlying predictors of fitting and adaptation
success throughout the hearing aid industry today (Chartrand, 2004b).
Another important reason for failure to fit is the lack of acknowledgment about
keratin and its vital role in maintaining homeostasis in the EAC, and in aiding in
adaptation of the otoprosthetics (Chartrand, 2004; Chartrand, 2003).
For these reasons and more, I submit that a study linking the presence or
absence of keratin to fitting challenges is crucial to the progress of the hearing aid field,
and for increased benefit to hearing impaired consumers. Such a study would draw out
practical applications that can be applied in dispensing practice, and provide guideposts
for accommodating the natural processes that might otherwise complicate fittings. It can
provide and meld, for the first time in the literature, a rich body of supporting evidence
from several related branches of study in the literature—physiological psychology,
47
neurophysiology, otoprosthetics, audiology, pharmacology, and hearing instrument
sciences—to add to the vast body of other hearing health literature. Finally, it will fill an
important gap in the knowledge base for hearing health providers, and reduce the delay
and subsequent failures so that greater numbers of hearing impaired consumers may
enjoy the benefits of today’s auditory rehabilitation cornucopia.
Statement of the Hypothesis
Is there a relationship between the keratin status of the external ear canal and
the resulting success rate of hearing aid adaptation? The non-directional null and
alternative hypotheses are, respectively:
1. Ho: Keratin status of the EAC has no positive relationship with successful
adaptation to hearing aids.
2. H: External ear keratin status is closely associated with success in adapting
to hearing aids.
Several ancillary questions were also addressed to provide a deeper explanation
of the above hypotheses, chiefly those dealing with the neurophysiology of the EAC, the
role and need for healthy keratin in the EAC, and measures for determining user fitting
or adaptation success with hearing aids.
Description of the Research Design
In this study, treatment conditions have not been manipulated beyond that of
everyday practice using best practice standards, nor were there controls to the study
group. For this and other reasons, this study was not a true experimental design
(Abrahams, 2005). Instead, it was a quasi-experimental or bivariate correlational study
that explores possible relationships between two variables—keratin status versus
48
adaptation—as they might be found in everyday clinical practice (Bordens and Abbott,
2002). Data was obtained from a retrospective file review of the experiences of hearing
aid patients where best practice standards were utilized during each fitting experience.
Timelines involved generally followed the sequela shown in Figure 6 (below).
Day
Initial Evaluation, Recommendation,
1
and Impression-Taking
Patient uses botanical solution for 10
day period while waiting for hearing
instrumentation
2-10
Delivery: Fitting, verification &
counseling for use of
Instrumentation
First Follow-up Visit: Post-fitting
measures, shell/ earmold
11
modifications or remake as needed
Second Follow-Up Visit: Post-fitting
measures, shell/earmold modifications or
remake as needed
14
Discharge evaluation
21
Figure 6. Typical timelines for sequela involved in dispensing tasks for this retrospective
study.
45
49
Operational Definition of Variables
In setting up this study, we quantified two main variables from the information
taken from sample files of participants. The predictor variable consisted of the keratin
status as visually observed on the surface of the EAC during video otoscopy in the initial
assessment. To quantify its status keratin thickness was rated on a Likert-type scale of
three levels as follows:
(1) Peeling or Absent Keratin
(2) Barely Visible or Thin Keratin
(3) Medium or Thick Keratin
The dependent variable consisted of physical fitting/adaptation success
judgments made again on a Likert-type rating scale, this time at five graduated levels.
Each of these denoted actions that were taken during the fitting process that may be
considered to signify difficulties (or lack of) in adaptation as follows:
1) Return of all instruments in a monaural or binaural fitting
2) Return one side of a binaural fitting for reasons of discomfort
AND/OR exchange model because of adaptation issue
3) Remake shells/earmolds for all of a monaural or binaural fitting
OR Remake shell/earmold for one ear in a binaural fitting
4) Unable to wear all day by end of initial two weeks but OK afterwards
OR Significant in-office modification as a result of complaint
5) Complaints of initial discomfort without need for modification OR
physical adaptation without any of the above over initial 45-day period
*Note: Multiple responses were rated to lowest level.
50
Both descriptive and correlational statistical analyses of these two variables were
analyzed, with emphasis on the Pearson product moment correlation r to demonstrate
over-all correlation between the two variables. Brought into the discussion elsewhere in
the paper will also be graphical depictions and data concerning subsidiary variables and
information relative to:
Age and gender of participants of this study
Type of instrumentation and fitting configuration
A description of the nature of the external auditory canal (EAC)
mechanoreceptors and neuroreflexes (Chartrand, 2005)
Credit return rates as reported by the Hearing Industries Association (HIA
data)
Variations in wearing schedule vs user experience (DigiCare, 2005)
Frequency of selecting certain models of instruments or earmolds (most recent
MarkeTrak data)
Demographic trends, such as age, sex, and degree of impairment (most
recent Aural Rehab Concepts data)
Frequency and effects of chronic conditions. Such as diabetes mellitus II
(DMII), gout, dehydration, neuropathy, vascular disease (Literature review)
Effects of commonly prescribed medications, as well as adherence issues
(Literature review)
Participants
Participants consisted of 98 adult hearing aid users (n=98) comprised of 62
males and 36 females, mean age 70.3 years, fitted between November, 2002 and
51
August, 2006. They were selected at random from a set of 435 files from a hearing
health and occupational therapy branch office located in southern Colorado. The ratio of
males to female participants is fairly representative of the proportion of older hearing
impaired individuals in the United States (Stabb, 1990). In addition, the median age of
70.3 years was fairly representative of those with permanent sensorineural hearing loss
in the general population (Gartecki & Erler, 1996). In all cases, all were fitted with
hearing aids utilizing the same standards of practice.
Materials
Materials consisted of several instruments that helped identify and verify
information from case history notes, video otoscopy observations, and other pertinent
data on each participant. Other instruments involved informed consent forms, medical
clearances and waivers, and other consumer disclosures.
Patient files of the selected participants. These contain detailed case and health
history notes, video otoscopy and audiometric data, completed instrument and earmold
order forms, programming notes and electroacoustic records for amplification, and a
running log of case notes per timeline protocols shown in Figure 6 above.
Participant Data Sheet onto which raw data information from each patient file
was transcribed by two File Reviewers (Exhibit A). The File Reviewers paid particular
attention to remake, repair, and return forms that might shed more light on the success
level of each case, striving to protect the identities of each participant. Interpretation of
data was spot-checked by each Reviewer to assure uniformity. The Lead Researcher
was not involved in this stage of data collection.
52
Rating Scale for Keratin Status and Hearing Aid Adaptation Success
(Exhibit B), which contained the data that would be reduced to two Likert-type scales for
rating data form the Patient Data Sheets. These sheets were filled out with a third
Reviewer involved to assure objectivity and accuracy of transfer data. Again, the Lead
Researcher was not involved with rating or compilation of file data.
HIPAA Informed Consent Form (Exhibit C), which allows informed consent of
communication between all entities involved, including between hearing health
professionals, physicians, lab, and factory personnel, and for research purposes. Even
though this was a file review study, each participant was made aware of the likelihood
that information from their case history would be used in a future study, and consented
to such.
Notice of Privacy Policy (Exhibit D), which provided a detailed explanation of
what was being referred to in the HIPAA Informed Consent Form. This form was posted
adjacent to the area where the Consent Form was signed and pointed out by front office
staff for the patient review at will.
In-house shipping and receiving logging records, which verify dates and
reasons for various actions recorded in patient files. These records were compared to
file notes to verify whether hearing aids were returned, remade, or exchanged.
Other materials were also used for gathering and analyzing data on ancillary
constructs and constructs as needed, such as appointment calendars and earmold
packaging labels. In addition, to assure compliance and uniformity in patient-assigned
activities (such as use of a botanical solution in the ear canal in preparation for being
53
fitted with hearing aids), written instructions were given each participant to take home
for future reference.
Procedures and Measures
After each patient file had been reviewed for accuracy, completeness, and
consistency, and the ninety-eight (n=98) participants were selected, the Participant Data
Sheet was used to record raw data from each patient file. In cases of RFCs, file
information was verified by comparing with the information recorded in the In-House
Logging Record. These data will then be reduced to a Rating Scale for Keratin Status
and Hearing Aid Adaptation Success. The Likert-type rating scales for keratin status
and hearing aid adaptation success were used and analyzed separately in their
descriptive form (i.e., expressed in range and middle tendencies). Then, the
combination of the two were be tabulated and analyzed in their relational or correlational
form.
During the preliminaries of this study, the question arose as to how many
levels of responses (or keratin status, in this case) would be needed to allow sufficient
differentiation. After practicing several rating scales, it was decided that seven, or even
five levels were impractical and do not allow enough visual differentiation of the levels of
keratin status for repeated observations. Therefore, it was decided to reduce the rating
levels to three levels as follows:
Level 1: Absent or peeling keratin
Level 2: Thin keratin with no or barely visible desquamation lines
Level 3: Moderate or thick keratin with easily discernable desquamation lines
54
For level one, it was decided that the end-results for absent or peeling keratin
had the same practical effect when wearing a hearing aid or earmold. In most cases of
peeling keratin, often caused by either DMII or mechanical removing of earwax (i.e.,
cotton swabs), the keratin is absent and not present to perform its role in maintaining
EAC homeostasis and shielding the mechanoreceptors of the neuroreflexes from over
sensitivity while wearing hearing aids or earmolds. At level two we found it difficult to
distinguish the practical effects of visibly present desquamation lines in thin keratin and
newly formed keratin that is still thin in appearance. A confounding issue with this level
was the high incidence of semi-dehydration seen in many of the older patients. We
decided to leave this confound in the mix simply because it reflects real life hearing aid
populations. Furthermore, it was noted that another confound, but one about which we
could do little, presented in this middle group, because use of the Botanical solution
appeared to improve keratin status during the fitting timeline, reducing the number of
modifications, remakes, and RFCs that otherwise might have presented in this
subgroup. The botanical solution usage could have been left off, except that—at least in
our practice—standardized use of it was considered part of recent best practice
standards (Gadd, 2006). Finally, at level three the practical effects of having moderate
or thick keratin appeared about the same. The differences may only reflect individual
characteristics (i.e., normal for a given individual, male or female).
The dependent variable (hearing aid adaptation success) was a little more tricky
in that qualitative data from case notes (post-fitting comments) must be associated with
quantitative actions taken (in-office modifications, factory remakes, returns for credit,
55
etc.). In this case, it was decided to utilize five levels of quantification. They were graded
as follows:
Level 1: Returned all fitted instrumentation for credit for failure to physically
adapt. This was presumably the worst of all possible outcomes, as it resulted in a
total failure to fit.
Level 2: Exchanged model to adaptation issue AND/OR returned one side of a
binaural fitting or remake of all fitted instruments. This was the next worst-case
scenario, but at least had the advantage of providing some help to the patient by
keeping one hearing aid.
Level 3: Remade shell/earmold for one side of a binaural fitting AND/OR unable
to wear all day by end of initial two weeks, but OK afterwards. While there may
also be non-adaptive reasons for these remakes, the overriding cause for
remake turned out to be adaptive.
Level 4: Significant in-office modification as a result of complaint AND/OR
Complaints of discomfort in ear(s). The same non-adaptive reasons mentioned
above applied somewhat to this level, but to a lesser degree.
Level 5: Physical adaptation without reports of the above over initial 30 day
period. Presumably, this would be considered the pinnacle outcome in a hearing
aid fitting.
To test both the null hypothesis or alternative, scaling of the variables required
that levels grew in magnitude in the same order: Absent keratin/failed trial to healthy
keratin/successful trial. In the event of more than one item being checked off in the file
review, the item with the lowest number prevailed. In other words, if “complaints of
56
discomfort” was checked off on the data sheet, and also “remade shell/earmold for one
side of a binaural fitting, the item with the lowest Level number was scaled for.
Data Processing.
Data from the Participant Data Sheet were transferred to the Keratin/Hearing Aid
Adaptation Scale for quantification. Participant data was summarized into univariate
descriptive statistics for gender, age, type of fitting, type of instrumentation, and other
data related to the study variables. These were expressed as means, ranges, standard
deviations, percentages and depicted in tables, graphs, charts, and within the text as
appropriate.
Data from the predictor and dependent variables (keratin status and adaptation
success) were expressed in correlational statistics using Pearson Product Moment to
express strength of relationship. To control for Type I error, a Bonferroni correction was
applied, along with a one-way ANOVA on the criterion variable(s) to assess whether
means were significantly different among participants.
For instance, in a trial run of n= 10 of file data using the measures outlined
above, a significant correlation was found r= .886, p<.01; F(1,8) = 29.254, p < .001. This
strongly supported the alternative hypothesis that there is a positive relationship
between keratin status of the external ear and the rate of adaptation success with
hearing aids. Of course, the larger n will help add internal and external validity to the
results. Also, the points of discovery relative to when sensitivities were considered
(before in the trial run and after the entire fitting process in this study) will make a
difference in outcomes. It was expected that this study will produce a far lower
correlation, albeit still significant enough to support the hypothesis.
57
Methodological Assumptions and Limitations
Each variable involved a number of related constructs, while potential confounds
were often tethered to the file reviewers’ ability to relate these constructs to written file
notations of each participant. To overcome this limitation, each reviewer was required to
take in-service training from the accredited materials of a CE course titled A Practical
Course in Neurophysiology and Video Otoscopy (IIHIS, 2004), and selected sessions of
the Licensing and Certification Exam Prep Course (IIHIS, 2006). The information in both
of these courses contains principles that are not common knowledge among dispensing
professionals, in general, regardless of academic preparation. For instance, keratin and
the neuroreflexes of the EAC are rarely, if ever, considered in current audiology
curriculum. Also, recognizing these factors as causal in hearing aid adaptation is still a
relatively new concept, and not readily acknowledged in the literature (Chartrand, 2004;
Chartrand & Chartrand, 2006).
A possible confound found in this study were the effects that having a study
would be expected to have on the results. For instance, the knowledge that the
researcher and associates possessed knowledge that there would be a study
conducted from these cases may have caused greater attention to detail during the
evaluation and fitting process, and also in more careful note-taking. This, admittedly,
may have had a positive effect on a lower rate of remakes and returns for credit (RFC)
that is normally not seen in a typical practice. Making up for a reduction in remakes and
RFC because of this artifact of the study was probably a higher rate of shell and
earmold modifications than would normally be seen in everyday practice. A more
proactive stance relative to these modifications, alongside more intensive patient
58
counseling, apparently avoided the level of failure fits that is more reminiscent in
everyday dispensing practice. A more practical approach to such a study would have
been to perform a retrospective study of another firm’s patient files. However, there
would likely have been no notations relative to keratin status, nor uniformity in file notes
notating reasons for failure to fit, such as financial motivations, auditory limitations, or
physical factors, such as over sensitive EAC neuroreflexes. In other words, there could
be no more objective study of these factors due to the fact that by including them would
likely produce the same results as evidenced by the two trial runs already performed on
two other sets of files.
In addition, at first blush it might appear that the sample size (N=98) is a
limitation. However, trial runs on the data (while refining the measures used in this
study) of N=10, N =18, and N =27 all produced stable results with little variation in the
before side of the rehabilitative process. Consequently, it was felt that n=98 represented
a strong sample relative to everyday practice. Otherwise, the design is relatively
straightforward and uncomplicated for purposes of replication as long as the above
precautions are observed. Instead, limitations of the current study tend to present in
Raters’ ability to discern patient motivation via file notes (financial vs comfort vs auditory
perceptions).
A limitation that may have limited generalization of outcomes in this study was
the standard practice of recommending the use of a natural botanical solution
manufactured by MiraCell, Inc. Because of positive benefits in preparing the ear canal
for wearing new hearing aids, each participant (as well as all hearing aid patients) was
recommended to use MiraCell in a manner that will be described in more detail in the
59
Discussion chapter of this paper. Though a sizable number of dispensers and
audiologists today consider recommendation of this solution as standard practice in the
best interests of new hearing aid users, not all do. So that, any replication of this
study—to realize comparable outcomes, anyway—would need to include use of this
solution in their fitting protocols.
Ethical Considerations
In this study, ethical considerations applied from several vantage points. From
the perspective of informed consent, disclosure of the auditory evaluation and
rehabilitation process was essential for positive participation by each patient (Corey,
Corey & Callahan, 2003). In other words, it was important that participants know that
their cases may be considered for research purposes, so that careful consideration be
given as to rehabilitative instructions. For uniformity in procedures depended highly
upon participant compliance.
Some observations were based upon patient reports and complaints during the
long process of evaluation, fitting, and post-fitting care (shown above in Figure 6). From
the vantage point of confidentiality, the new HIPAA regulations required us to specify
those with whom patient information could be shared. This included the auditory rehab
team, referred or referring physicians and others, lab technicians, and research
personnel. Therefore, our posted Notice of Privacy Practices includes, under “Other
Permitted Uses and Disclosures without Your Authorization”, the following statement,
Research. We may use or disclose your health information for inhouse research projects. If such research is published it will be done
without personal identifiers. In some cases, an Institutional Review Board
60
may approve a waiver of authorization for use of disclosure when
appropriate.(DigiCare, 2003).
Other considerations relative to informed consent involved the following routine
clinical activities:
Intake interview
Case history questions
Video otoscopy remarks
Tests for neuroreflex location and sensitivities
Audiometric and functional speech tests
Hearing aid/earmold recommendation
Otoblock insertion
Impression taking
Delivery instructions
Prosthetic fitting procedures
Digital programming and outcomes verification
Earmold/shell modifications
Post-fitting evaluations and patient reports
Personal ear care instructions
Cerumen management, when indicated
Without thoroughly and consistently addressing these informed consent issues,
experience has shown that patient perceptions over physical responses like cough or
gag reflexed evoked during otoblock insertion or occasional superficial surface capillary
bleeding during impression-taking may be construed as professional carelessness or
61
negligence. Ethical dilemmas typically involve differences in perceptions between
patient and attending professionals (Chartrand, 2004a).
If this study had been designed as a blind or double-blind (proactive) study, an
ethical dilemma would have presented as a conflict between informed consent and
objectivity. Furthermore, such a study would have by necessity included withholding
treatment (for control purposes), which would have abrogated rules of ethical conduct in
human research (Bailey and Schwartzberg, 1995). Consequently, these dilemmas were
resolved by making the study a retroactive case file study, where informed consent and
objectivity were satisfied by compiling data on cases that have already occurred. In
addition, patient identity was protected by being referred to only by case number during
the file transcription process.
A limitation that proved to be somewhat of an ethical dilemma resulted as an
unintended consequence of the 30-day trial requirement in the State of Colorado
hearing aid consumer regulations (Audiology and Hearing Aid Providers Registration,
2002). Such regulations, though intended to protect consumers from unscrupulous
business practices, are at odds with the rehabilitative process required in successful
auditory rehabilitation. For they force unrealistic expectations upon consumers (and, by
extension, the regulating agencies who receive more consumer complaints arising
therefrom) who need more time to overcome the neurological and psychosocial effects
of auditory sensory deprivation. In the vast majority of long-standing cases, a 30-day
period is simply not long enough. On the other hand, an even longer period promotes
untenable financial risks for dispensing professionals (Waters, 2003). We believe the
state should leave it up to consumers and hearing health professionals as to
62
expectations, timelines, and financial considerations. In this particular study, this
artificial barrier caused some patients to give up before neurological adaptation
occurred, which effectively eliminated them from the study. Furthermore, when outcome
expectations are unrealistically high and consumers give up too early, it often
discourages them from seeking further help for their problem. Such failures place undue
cost and trust pressures on an industry already beset by universal misperceptions over
the nature of hearing impairment (Sandlin, 1996).
The University’s Institutional Review Board (IRB) reviewed all ethical aspects in
our application and found them acceptable and within ethical bounds. Furthermore,
every precaution has been taken not to disclose the identity of any of the participants,
including from the Rating Reviewers who rated the data gleaned from patient files.
63
CHAPTER FOUR
RESULTS
Overview
The research question described in the preceding chapter focuses upon the
possible correlation between keratin status of the external ear and participant success
in physically adapting to wearing hearing aids. The predictor variable of keratin status
was observed during the initial evaluation of each participant and recorded in three
status levels: 1) absent or peeling keratin, 2) thin keratin with no or barely visible
desquamation lines, and 3) moderate or thick keratin with easily discernable
desquamation lines.
The dependent variable is the demonstrated ability to adapt physically to hearing
aids over a period of approximately 45-days—during which time various evaluation,
fitting, and post-fitting tasks were performed. The ability to adapt was observed and
graded at five levels, summarized as: 1) Returned all fitted instrumentation for credit, 2)
exchanged model due to adaptation issue and/or returned one side of binaural fitting or
remake of all fitted instruments, 3) remade shell/earmold for one side of a binaural fitting
and/or unable to wear all day by end of initial two weeks, but OK afterwards, 4)
significant in-office modification and/or complaints of discomfort in ear(s), 5) successful
physical adaptation without difficulty or modifications over initial 30 day period.
Although this was a retrospective archival study, the researcher and associates
have made every possible effort to assure uniform protocols and practice standards,
including completion and consistency of file notes and task documentation. Files
indicating financial or auditory-based reasons for failure to fit were excluded from this
64
study. Consequently, only those whose fitting results pertained primarily to fitting
comfort and physical wearing of hearing aids were included. Data compiled from the
Keratin/Adaptation Rating Forms can be found in Appendix E.
Description of Participants
Of the 98 adult participants (n=98), 63.2% were males and 36.8% were females.
The mean age for males was 70.73 years (SD=10.41), and the median age was 62.0
years of age (range 51.0 years). For females, the mean age was 69.55 years
(SD=16.04), and the median age was 71.5 years (65.0 years). Together, the mean age
was 70.29 (SD=12.75), median age 70.50 years and the range was 66.0 years
(minimum 29, maximum 95). All participants were fitted with new hearing instruments
between November, 2002 and August, 2006. See Table 2 below for more summarized
participant data. Figure 7 (below) presents a frequency histogram of the age of
participants.
Table 2
Descriptive Analyses of Participants
Measure
Sample size n/N(%)
Males
Females
Both
62(63.2%)
36(36.8%)
98(100%)
Minimum Age
44
29
29
Maximum Age
95
94
95
Range
50.0
65.0
66.0
Mean
70.73
69.55
70.29
69.5
61.5
62.00
10.41
16.04
12.75
Median
Standard Deviation
65
Skewness
Kurtosis
-.021
-.910
-.696
.093
.427
.983
20
Frequency
15
10
5
Mean =70.2959
Std. Dev. =12.74509
N =98
0
20.00
40.00
60.00
80.00
100.00
Age
Figure 7. Frequency histogram showing the distribution of age among study
participants.
66
Participant Fitting Data
Approximately 57.14 percent of the participants were fitted binaurally, while
42.8% were fitted monaurally (see Figure 8 below). Anecdotal observations by file data
raters noted that reasons for being fitted monaurally or binaurally pertained mostly to
type and degree of hearing impairment; however some decided on monaural fittings for
financial or personal preference reasons. No data was compiled on this aspect.
Of the type of instrumentation fitted, 69.18% were custom instruments (full-shell,
canal, or mini-canal) and 30.82% were post-auricular (BTE) fittings (see Figure 9
below). None were completely open-ear fittings; however, several were IROS or CROSstyle venting configurations. Type of instrument was chosen mostly because of the type
and degree of hearing loss, but also from personal preference, such as cosmetics,
dexterity, comfort, and factors from past amplification experience. The vast majority of
participants were first time users (72%), while 28% were previous hearing aid users
(see Figure 10 below). The reader will note that the proportion of post-auricular (BTE)
users (30.82%) is considerably higher than the industry average (18-22%) during the
time of the fittings for this group of participants occurred (Hearing Industries
Association, 2006).
67
70
60
50
%
40
30
20
10
0
Males
Females
Both
Monaural
37.09
52.77
42.86
Binaural
62.91
47.22
57.14
Figure 8. Monaural vs binaural fittings.
80
70
60
50
% 40
30
20
10
0
Males
Females
Both
Custom
66.13
72.23
68.37
BTE
33.87
27.77
31.63
Figure 9. Custom instruments vs post auricular (BTE) instruments.
68
70
60
50
%
40
30
20
10
0
Males
Females
Both
Previous Users
30.65
38.88
33.67
New Users
69.35
61.11
66.33
Figure 10. User Experience: Previous vs new users
Table 3
Descriptive Analysis of Participant Fitting Data
Measure
Males
Females
#
%
#
Sample size n/N
62
63.20
36
Monaural Fitting
23
37.09
Binaural Fitting
39
Custom Instruments
%
Both
#
%
36.80
98
100.00
19
52.77
42
42.80
62.90
17
47.22
56
57.14
40
64.51
22
61.11
67
68.36
Post-Auricular (BTE)
32
51.61
9
25.00
41
41.83
Previous Users
19
30.65
14
38.88
33
33.67
New Users
43
69.35
22
61.11
65
66.33
69
Participant Fitting Experience Data
Overall, 5.1 percent of patients returned all of their hearing aids for credit (RFC),
while 13% of patients experienced a complete remake of shells or earmolds because of
fitting discomfort issues. There was some overlap between RFCs and remake, as
almost all RFCs, due to rapidly developing discomfort issues, required a shell/earmold
remake before eventually becoming an RFC. More importantly, 51% of all participants
required in-office modification of hearing aid shells or earmolds due to
comfort/adaptation issues (see Figure 11 below). Reasons for modifications ranged
from fitting discomfort due to acclimatization and genuine fitting problems to
insertion/removal and dexterity challenges. Cases of remakes that resulted from
acoustic feedback or other acoustic issues were excluded from the study. See Table 4
below for more data.
60
40
%
20
0
Males
Females
Both
Remakes
16.13
8.33
13.01
RFCs
6.45
2.78
5.11
Modifications
51.61
50.01
51.02
Figure 11. Remake, RFC, and in-office modification rates.
70
Table 4
Descriptive Analysis of Remake, Return-For-Credit (RFC), and In-Office Modification
Experience Data.
Measure
Males
Females
Both
#
%
#
%
#
%
Sample size n/N
62
63.20
36
36.80
98
100.00
Remake Yes
10
16.13
3
8.33
13
13.00
Remake No
52
83.87
33
91.66
85
86.73
RFC Yes
4
6.45
1
2.78
5
5.10
RFC No
58
93.54
35
97.22
93
94.89
Modifications Yes
32
51.61
18
50.00
50
51.02
Modifications No
30
48.39
18
50.00
48
48.98
Keratin Status Data
Keratin status was observed via video otoscopy during the initial evaluation,
and as stated in the hypothesis, was one of two key variables explored in this study. At
Keratin status level 1, we found 22.58% of males exhibited absent or peeling keratin in
the EAC, while only 11.11% of females exhibited same. At level 2, the proportion is
33.87% of males exhibiting thin keratin with an even larger 52.77% of females at this
level. Finally, at level 3 we found a more even distribution between the genders with
43.54% of males and 36.11% of females exhibiting medium or thick keratin in the EAC.
Together, the distribution was 18.36%, 40.81%, and 40.81% at levels 1, 2, and 3,
71
respectively (see Figure 12 below). As shown in Table 5 (also below), the majority of
ratings for males, females, and both genders, fell at the high end of the status levels
causing a negatively skewed distribution.
60
40
%
20
0
Males
Females
Both
Level 1
22.58
11.11
18.37
Level 2
33.87
52.77
40.81
Level 3
43.54
36.11
40.82
Figure 12. Keratin Status at each level of condition at initial evaluation
Table 5
Descriptive Analyses of Keratin Status.
Measure
Males
#
Sample size n/N
62
Females
%
63.20
#
36
%
36.80
Both
#
98
%
100.0
72
Keratin Level 1
14
22.58
4
11.11
18
18.37
Keratin Level 2
21
33.87
19
52.77
40
40.81
Keratin Level 3
27
43.54
13
36.11
40
40.81
All Levels Status
Mean
2.21
2.50
2.22
.10
.11
.08
Lower Bound
2.01
2.01
2.08
Upper Bound
2.41
2.47
2.37
5% Trimmed M
2.23
2.28
2.25
Median
2.00
2.00
2.00
Variance
.627
.42
.55
SD
.792
.649
.739
Minimum
1.00
1.00
1.00
Maximum
3.00
3.00
3.00
Range
2.00
2.00
2.00
Interquartile R
1.00
1.00
1.00
Skewness
-.39
-.29
-.39
Std. Error
.30
.39
.24
-1.29
-.61
-1.08
.59
.77
.48
Std. Error
95% Confidence
Interval for Mean
Kurtosis
Std. Error
73
Adaptation Experience Ratings
As stated earlier, adaptation levels were rated with five levels, and were based
upon participant experiences relative to adapting to shell and earmold couplers of their
hearing instrumentation. At level 1, which includes those who returned all of their
hearing aids for refunds (RFC), we find 6.45% of males and 2.48% of females. At level
2, where the instruments were exchanged for another model or where one of the
instruments in a binaural set were returned, we find 9.68% of males and 0% of females.
At level 3, where shell/earmolds were remade for one side of a binaural fitting and/or
they were unable to wear all day by end of initial two weeks, but OK afterwards, we find
8.82% of males and 13.89 of females. At level 4, where significant in-office
modifications were required and/or there were complaints of discomfort in ear(s), we
find 27.42% of males and a much larger 36.11% of females.
And, finally, at level 5, where there was successful physical adaptation without
difficulty or modifications over the initial 30 day period, we find an almost even 48.39%
of males and 47.22% of females. For both genders together we found 4.08%, 6.12%,
10.20%, 30.61%, and 47.96% at levels 1,2,3,4, and 5, respectively (see Figure 13
below). As seen in Figure 14 (below), the majority of cases are skewed (negatively)
toward the high end of the adaptation spectrum. See Table 6 below for more detail.
74
50
45
40
35
30
% 25
20
15
10
5
0
Males
Females
Both
Level 1
6.45
2.78
5.11
Level 2
9.68
0
6.12
Level 3
9.68
13.89
11.22
Level 4
27.42
36.11
30.61
Level 5
48.39
47.22
47.99
Figure 13. Distribution of adaptation levels among participants.
75
50
Frequency
40
30
20
10
Mean =4.1224
Std. Dev. =1.09606
N =98
0
0.00
1.00
2.00
3.00
4.00
5.00
6.00
Adaptation
Figure 14. Histogram showing distribution curve of the levels of hearing aid adaptation
among study participants.
Table 6
Descriptive Analyses of Adaptation Experiences of Participants.
Measure
Sample size n/N
Males
Females
Both
#
%
#
%
#
62
63.20
36
36.8
98
%
100.0
76
0
Adaptation Level 1
4
6.45
1
2.78
5
5.11
Adaptation Level 2
6
8.82
0
0.00
6
6.12
Adaptation Level 3
6
8.82
5
13.89
11
11.22
Adaptation Level 4
17
27.42
13
36.11
30
30.61
Adaptation Level 5
30
48.39
17
47.22
47
47.99
Total Adaptation
Mean
4.05
4.25
4.12
Std. Error
.15
.15
.15
95% Confidence
Interval for Mean
Lower Bound
3.75
3.94
3.90
Upper Bound
4.35
4.56
4.34
5% Trimmed M
4.16
4.34
4.24
Median
4.00
4.00
4.00
Variance
1.42
.82
1.20
SD
1.19
.91
1.09
Minimum
1.00
1.00
1.00
Maximum
5.00
5.00
5.00
Range
4.00
4.00
4.00
Interquartile R
1.25
1.00
1.00
-1.17
-1.51
-1.30
.39
.39
.24
.39
3.22
1.06
Skewness
Std. Error
Kurtosis
77
Std. Error
.60
.77
.48
Relationship between Keratin and Adaptation
As stated earlier, the two main variables in which we were interested were
keratin status and the rate of adaptation success. Since it was not possible within our
design to attribute causality, our primary purpose was instead to discover what happens
to the dependent or criterion variable (success rate of physical adaptation to wearing
hearing aids) when the predictor variable (keratin status) was changed status levels.
The number of rating levels, though incrementally differing for each variable, was
decided upon on the basis of what could be expected on a test-retest basis in
differentiating and quantifying the condition of each variable. For instance, the predictor
variable (keratin status) could only be judged via video otoscopy assessment; while the
criterion variable (adaptation success) could only be judged on the basis of written
records of patient experiences. Then, upon uniform interpretation of what these records
implied, data was quantified on the scale set forth on the adaptation rating scale
described above.
To test the relationship between keratin status and adaptation success, linear
regression analysis was conducted, from which Pearson correlation coefficients were
computed for the two variables. To control for Type I error, a Bonferroni correction was
applied, which showed that correlation was significant at the <0.01 level (2-tailed). The
correlational analysis showed that the relationship between keratin status and
adaptation was statistically significant (r = .627, p<.01; F(1, 96) = 62.200, p <.01.).
Therefore, the hypothesis that there was no effect between these two variables was
78
rejected at the <0.01 alpha level, affirming the alternative hypothesis that the status of
EAC keratin shares a strong relationship with hearing aid adaptation success.
Furthermore, to test the relationships of keratin status with other variables at the
<0.01 alpha level, multiple linear regression was conducted. In Model 1 of the analysis
were included the predictor variables of remakes and returns for credit (RFCs), which
are actually subset indicators for the criterion variable adaptation. The results of this
analysis indicated that remakes and RFCs shared significant relationship with keratin
status, R2 change = .417, F(2, 95)= 10.015, p< .01.
Separately, remakes and RFCs—in linear regression analysis—indicate negative
correlations with keratin status, r = -.372, p<.01; F(1, 96) = 15.425, p<.01 and r= -.386,
p<.01; F(1, 96) = 16.793, p<.01, respectively. Since the ratings of these variables were
not scaled to show positive direction as was the main variable adaptation, the results
indicate that as the status of keratin levels decrease, both remakes and RFCs increase.
As was noted earlier, all RFCs resulted in the keratin status level 1 group, while
remakes were consequently spread over keratin status levels 1, 2, and 3.
Analysis was conducted for the remaining variables included in the Model 2 data
set. Included in this subset were age, gender, fitting configuration, and type of
instrumentation as predictor variables, and keratin status as the criterion variable.
These measures indicated varying degrees of significant and insignificant influence with
keratin status, R2 change = .515, F(6,91) = 5.469, p< .01.
Separately, the direction of relationship with specific variables is better indicated,
with age, gender, and type (r = .089, r = .026, and r = .031) showing an insignificant
relationship with keratin status, and fitting (r = -.219) indicating a mild or insignificant
79
negative relationship. See Appendix F for complete SSPS (GP 14.0) calculations for the
above results.
80
CHAPTER FIVE
DISCUSSION
Summary of Findings
Within the context of the participants of the current study, the findings
demonstrate a strong relationship between EAC keratin status and hearing aid
adaptation success. Therefore, based on the data, the beginning status of a
participant’s EAC keratin layer appeared to have significant bearing on their ability to
adapt successfully to wearing hearing aids. In turn, the sub-criterion variables—
remakes and returns-for-credit (RFCs)—demonstrated a negative correlation with the
level of keratin status. In other words, as keratin status improved, the rate of remakes
and RFCs tended to decline. These are important findings for an industry, which
currently does not recognize the crucial role that EAC keratin can play in not only
reducing inordinately high remakes and RFCs, but also in assuring comfort and
acceptance of the fitted instruments. By extension, the constructs of the current study
should be included in consumer self-assessment inventories and surveys, such as the
Abbreviated Profile of Hearing Aid Benefit (APHAB), Satisfaction with Amplification in
Daily Life (SADL), and the International Outcome Inventory for Hearing Aids (IOI-HA).
Furthermore, the evidence of this study points to the need for more continuing
education training in video otoscopy biomarker assessment for both entry level and
experienced hearing care professionals, as well as the development of protocols for
patient counseling in optimal ear care for all sectors of the dispensing community.
Throughout this chapter this investigator will discuss the implications of this
study. Internal and external validity issues, as well as limitations, will also be discussed.
81
More importantly, I will attempt to lay down the rationale and foundation for a
continuation of this work, so that the industry may gain a better understanding of the
importance of EAC keratin status and factors affecting it, especially in terms of
mitigating factors that positively impact auditory rehabilitation. I will begin with a brief
discussion of the findings, and proceed into specific areas of special consideration.
Illustrations will provide further explanation for discussion. Also, there will be a
discussion about the future directions of needed research relative to the issues and
constructs raised in this paper.
Explanation of the Findings
In the current study, the age of the participants (29-95) did not share a significant
relationship with either the predictor variable (keratin status) or criterion variables
(remake, RFCs, or adaptation). Participants’ mean age (70.29 years) was typical for
most private hearing aid dispensing practices in the United States (Peterson and Bell,
2004). Moreover, with removal of extraneous issues—i.e., dexterity and cognitive
limitations—age also did show a significant relationship with the comfort factors in
wearing earmolds and hearing aids.
In the gender domain, however, some interesting differences were indicated in
the data. Some of these will be discussed at more length further in this paper. In
summary, however, the gender factor appeared significant in the:
fitting configuration (males tended to need more binaural fittings than females)
type of preferred instrumentation (females tended to choose more behind-theear instruments)
82
remakes and RFCs (males experienced roughly twice the rate of remakes and
RFCs as females)
degree of keratin thickness and status (males, while generally exhibiting
thicker keratin, also exhibited higher incidence of abnormal keratin status)
ability to adapt successfully to wearing hearing aids (at the lower keratin status
levels, males tended to experience more failures to fit, but at the higher levels
of adaptation both genders adapted about the same)
Regarding amplification experience, an unusual finding was that there were twice
as many new users as there were previous users. This is upside down from other
studies’ findings in general, where penetration into the non-user market has slowed
dramatically in recent decades and previous users increasingly make up a larger
majority of those fitted each year in the United States (Strom, 2002). For instance, in a
typical practice today, less than 45% of fitted users are new users, whereas more than
two-thirds of participants in the current study were new users. One possible explanation
for this remarkable difference may have to do with the type of consumer education,
rather than conventional advertising, this particular offices uses to promote for new
patients. Also, the proportion of new users may have been positively impacted by the
fact that this facility was the first full-service hearing care facility in the region.
It is even more remarkable, however, that it is usually the new users that are the
most difficult to satisfy than patients with prior amplification experience (Yanz & Olson,
2006; Kochkin, 2000; Chartrand, 1999). Yet, the rate of credit returns for new-user
participants of this study were significantly below the norm (Mims-Voll and Jones,
1998). RFCs were also little less than what has been reported in annual dispenser
83
surveys, which typically show only about 25-30% of that reported by their
manufacturers. One explanation of why dispensers so dramatically underreport credit
returns is that they tend to count only cases for which they have had to make a full
refund. More typically, dispensers in self-assessment surveys do not count instruments
that were sent back for same-brand model exchanges or even complete changes of
brand names, or instruments ultimately rejected in multiple hearing aid trials for the
same patient, a practice that still lingers on from the Carhart days (Ross, 2004). These
factors, of course, distort real world accounting from the manufacturers’ point of view,
and markedly understate a serious problem in hearing healthcare today (Strom, 2005).
In the case of this study, however, the manufacturing and dispensing entities were
nearly always one and the same (i.e., the dispensing office’s headquarter facilities
included hearing aid and earmold manufacturing). So, that reporting of credit returns for
the purpose of this study included every device returned regardless of reason, except
those relative to financial, dexterity, or cognitive reasons (these were counted in regular
reporting, but excluded from this study).
As was earlier noted there were nearly twice as many males as females in the
study. This correlates closely with studies showing that there are approximately twice as
many older adult males as females with serious hearing impairment (Staab, 1990).
Other gender-based observations involved the fact that males tended more toward
binaural hearing than females. This is likely due to the fact that hearing loss in males is
often more severe and longer-standing than for females fitted with hearing aids.
Therefore, by the time hearing impaired males typically seek help for their hearing loss,
84
the need for amplification is substantially greater, explaining the more pronounced need
for binaural rather than monaural amplification (Chartrand, 1999).
At the time of this study, the proportion of sales of BTE style instrumentation
(versus custom instrument styles) in the general market was about half what it is in
2006, yet a higher proportion of females in this study tended to choose the BTE style
than did males. This may seem odd in light of the fact that BTE devices are generally
stronger in terms of potential gain and output than custom instruments relative to the
hearing impairments they are designed to accommodate. Since females tend to have
milder losses overall than males, especially at their first hearing aid fitting, the only
plausible explanation for their choice of BTEs would have been that females felt less
conspicuous wearing an instrument behind their ears where hair can cover the
instrumentation. Added to this is the fact that BTEs, especially for the milder losses,
involve less physical intrusion into the EAC than do custom instruments. In these cases,
an open style or minimal contact earmold is usually the coupler of choice. These factors
may have also influenced the more positive user experiences of females. Conversely, in
males choosing the less-powerful custom instruments for more serious hearing losses—
especially severe in the high frequencies in most cases—they may have set themselves
up for more fitting difficulties, such as feedback oscillation, more discomfort issues, and
realization of the gain and output required to reach worsened high frequency thresholds.
This fact may also explain why males experienced more adaptive challenges overall,
such as in remakes, RFCs, and in-office modifications. Also we may consider the
finding that male participants—who otherwise tend to exhibit thicker EAC keratin than
females—demonstrated more level 1 keratin (absent or peeling) anomalies. It is entirely
85
possible that this unexpected anomaly resulted from more frequent and aggressive use
of cotton swabs in personal ear care, and use of hypertensive medications and other
contributors.
External Auditory Meatal Keratin Influence
As demonstrated in the earlier literature review, a healthy auditory canal exhibits
normal skin keratinocytes from the region of the tympanic membrane and throughout
the canal lumen (Johnson & Hawke, 1988). This outer layer is necessary for the
maintenance of homeostasis and self-cleaning properties of the human ear canal
(Chartrand, 2004). The absence or disturbance of this layer of inorganic tissue may set
up the EAC for more chronic ear problems, such as external otitis, chronic itching,
hypersensitivity of mechanoreceptors which activate the EAC reflexes, and, in turn,
complicate even the best efforts during hearing aid adaptation (Chartrand & Chartrand,
2006; Robert, Funnell & Lazlo, 2005; Persaud et al., 2004).
As will be explained later on, one of the challenges faced by this investigator in
designing this study was in developing reliable biomarkers that can be utilized during
video otoscopic examination in any practice setting. These biomarkers were graded into
three classifications or levels of keratin status, levels 1 through 3, from absent/peeling to
a thick layer of keratin. Those at level 1 were expected to exhibit the greatest difficulty
in adapting to hearing aid earmolds, regardless of age or gender, and the data
somewhat supported that expectation. In other words, all of those who failed to
complete the fitting process exhibited level 1 keratin status; but by far not all those who
exhibited level 1 failed to fit. From file notes it appears that the use of the recommended
Miracelltm botanical solution and/or cessation of causal factors allowed the majority of
86
the level 1 group to improve their keratin status during the 45 day process until they
succeeded at various levels of adaptation success. To reiterate, level 1 consisted of
cases where the keratin layer was not visible via video otoscopy or which was peeling
from the underlying epithelium. About one fifth of participants presented at their initial
evaluation at this level. Causes for absent and/or peeling keratin have been identified as
use of cotton swabs or caustic solutions (e.g., boric acid, hydrogen peroxide, etc.),
semi-dehydration, prescription medication side-effects (e.g., diuretics, vasodilators,
antihistamines, anti-inflammatories, etc.) and/or underlying disease (e.g., diabetes
mellitus II, gout, acidosis) ((Heilbrun, et al., 2003; Roland, 2002; Wrong Diagnosis,
2007). See Figure 15 below for an example of two cases of peeling keratin. The case on
the left shows a case where the keratin had been disturbed by mechanical trauma (a
Bobby pin, in this case). The case on the right was of a newly diagnosed case of
diabetes. The differentiating factor for the clinician is that the left otoscopic view shows
shiny desquamation lines of newly formed keratin coming up from beneath the peeled
keratin. The view on the right has no such lines, as the systemic ability to produce
keratin has become inhibited by a rapidly developing case of insulin-dependent diabetes
mellitus II (DMII). Both cases are considered essentially subclinical and do not actually
require medical referral under the FDA 1977 Hearing Aid Rule. However, the diabetic
case was referred for medical examination. This case underlines the need for ease-ofaccess and cooperation between health professions. Furthermore, the mechanical
trauma case, once healed, was able to adapt successfully to their new hearing aids
without difficulty, while the diabetic case had complications requiring extensive
modifications of their hearing aid shells before comfort could be realized.
87
Figure 15. Two cases of peeling EAC keratin. The case on the left is of an example of
mechanical trauma; the case on the right is of one with rapidly developing DMII. Only
the case on the left shows new keratin “lines” coming up underneath the peeled layer.
Figure 16 (below) provides two otoscopic views of the human EAC. One is of a
case without any visible signs of keratin (i.e., level 1 status). Such cases typically
experience neuroreflex activity activated by the slightest pressure of the earmold. In
some cases, the passage of a short period of wear (i.e., 30 minutes) can cause
hypervascularization at the TM or trigeminal reflex. This can cause increased need for
gain and output accompanied by own voice resonance difficulties. The other case
shown in Figure 15 is of a case exhibiting a thick layer of keratin (i.e., level 3 status).
The data show that these cases demonstrated the highest level of adaptation to hearing
aids and earmolds without remakes or extensive in-office modifications. More otoscopic
views are displayed in Exhibit G.
88
Figure 16. Two cases: Absent keratin (L), thick keratin (R).
The data from the current study showed that all cases of remakes, RFCs, and
failure to fit came from those that exhibited level 1 keratin status at the initial evaluation.
However, as stated earlier, many level 1 participants went on to grow back normal
keratin and eventually adapted to their hearing aids or earmolds, possibly due to use of
the recommended solution. Ideally, it would be best practice standards, as well as
evidence based practice (EPB), to follow the progression of these cases in routine
hearing health practice (Chartrand & Chartrand, 2006).
Level 2 involved thin or medium thickness keratin. No known pathologies or
causes have been observed to affect this status level by this investigator, although
some level 2 cases may be recovering level 1 cases. It is assumed, by way of repeated
empirical observations, that females typically exhibit thin or medium keratin. The
hypothesis that females exhibit thinner keratin than males was not tested in this study.
89
However, more than half of female participants fell into this category, whereas only a
third of male participants did. It may also be that normal keratin formation is an
individual characteristic. Empirical observations have indicated that keratin grows at
about the same rate as hair and nails (about 1 mm per day), but we know those rates
vary widely from individual to individual (Chartrand, 2005). Conversely, males exhibited
a larger proportion of thick keratin (level 3) status than females. At these levels (2 and
3) it may be assumed that this state is normal, which would partially explain why the
correlation with adaptation, though strong, was not absolute.
Furthermore, in examining keratin levels with levels of hearing aid adaptation,
those who failed to fit indicated it was due to discomfort/adaptive reasons. As expected,
males—who also made up most of the level 1 of keratin status—comprised threequarters of those failing to fit (e.g., complete return of hearing aids at the end of the
fitting process). At level 2 of adaptation, characterized by those who experienced partial
returns and/or remakes, males made up all of that group, with no females represented.
Level 3 of adaptation, characterized by extensive in-office modifications, was most
heavily represented by female participants. This would point to a tendency to require
more modifications for reasons of discomfort in females due to thinner keratin layer,
even without extraneous or underlying causes. Levels 4 and 5 of adaptation (some
modifications and successful adaptation without modifications) found roughly equal
numbers of male and female participants, or about three-quarters of all participants.
Therefore, the relationship between keratin status and adaptation success appear
strongly related, though causation—due to non-experimental study design—could not
be established.
90
Use of Video Otoscopy
A thorough search of the literature will show that the present study represents
a pioneering effort. It brings together information from various related healthcare
fields—neurophysiology and behavioral science, audiology and hearing instrument
sciences, otolaryngology, dermatology, and others—to infer relationships that require
examination of a wide swath of data. Likewise, several branches of technology
advancements—video otoscopy, otoprosthetics, hearing instrument manufacturing, skin
care, and an array of advanced micro-topographic mapping technologies—have come
together so that this study’s hypothesis could be investigated objectively. In other
words, it is doubtful, given the fortuitous serendipity of the foregoing, that this
hypothesis could have been easily investigated until now. Since no previous studies
could be found that had examined the impact of keratin status upon hearing aid or
earmold adaptation issues, this investigator and associates were faced with challenges
in the effort to anchor the constructs of the study to solid replicable procedures and
measures.
In addition, video otoscopy technology in clinical practice was relatively new
(Chartrand, 2003b). Until 1992, that which was commercially available was prohibitively
expensive, and involved endoscopy and surgical video otoscopy, which were limited to
use in surgical, educational and research purposes (Sullivan, 1995). In 1992, this
investigator was involved with investigating the practical applications of the first two
commercially available units. Hence, I worked toward establishing training and user
protocols for professional dispensers worldwide. These early units were manufactured
by Jed-Med (St. Louis, MO) and distributed by Starkey Laboratories, Inc. (Eden Prairie,
91
MN) under the label StarMedtm. During those early days, we had to work with new
considerations in color, lighting, and image clarity to develop consistency and accuracy
for differentiation of otophysiology and otopathology (Curtin, Wolfe, & May, 1982). For
purposes of everyday clinical application, the standard by which these outcomes were
measured was the Welch-Allyn Fibre Optic hand-held otoscope, which was considered
the standard at the time.
Today, thousands of dispensing, audiology, and medical professionals use video
otoscopy as a superior alternative to hand-held video otoscopy (Chartrand, 2003b;
Trace, 1996). Not only does video otoscopy projected onto a high-definition color
monitor provide a much more definitive view, but the technology has dramatically
increased our knowledge of human EAC behavior. It has made detection and referral of
Red Flag cases accurate and more conclusive (Mbao, Eikelboom, Atlas & Gallop,
2003). However, this investigator feels that current use of video otoscopy in dispensing
practice still falls far short of its potential value for benefiting the hearing aid fitting
process. More expedient use of the technology may assist in (Chartrand, 2003b; Trace,
1996):
The assessment of EAC biomarkers, such as keratin status, neuroreflex
sensitivity, and structural anomalies relevant to idiosyncrasies affecting
successful hearing aid fitting and auditory rehabilitation protocols
Providing objective, recordable patient file documentation. Until the advent of
video otoscopy, it was pretty much one professional’s word against another’s.
With video otoscopy, the real-time image is projected onto a high-definition
color monitor for all to see. Also, the printed record avoids unnecessary debate
92
and fosters cooperative teamwork between allied professionals. See figure 17
below.
Video otoscopy informs the consumer as to the status of their external ear.
They may be counseled relative to appropriate personal ear care protocols,
and better understand factors that are otherwise out of the control of the
professional when outcomes are not what they want them to be.
Improvements in EAC may be documented as the hearing aid patient follows
the recommendations of their hearing care professional, encouraging them to
go forward in achieving success in their quest for better communicative health.
Figure 17. MedRx Video otoscope (used by permission from MedRx, Inc.)
93
Internal and External Validity Issues
As discussed in the foregoing, one of the difficulties in designing this study
was the lack of already established and recognized tools of measurement. Hence, I had
to rely upon my experience in examining and working with thousands of hearing
impaired patients over the past three decades. Nearly 15 years of that time involved
video otoscopy, which has provided some bases for understanding the constructs of this
study. For instance, one important construct was the role and purpose of keratin in the
EAC. While the otolaryngology, oncology, and dermatology fields have published a
plethora of studies and documentation about keratin, the dispensing and audiology
fields have almost totally shunted aside such considerations. The challenge was to
devise measures that have not previously existed, and which could be replicated by
trained clinicians, and which would stand up under the rigors of scientific scrutiny. I feel
these objectives were accomplished with the incremental identification of the 3 levels of
keratin status used in this study.
Moreover, measures for determining levels of adaptation were much easier to
devise, because the persistent problems of excessive remakes, in-office modifications,
and returns for credit are well understood. Their very excesses in industry data point to
a profound failure to fit. So do the data that reveal lack of market penetration and
negative consumer and regulatory perceptions—unfair or not—which continue to
hamper the dispensing field’s ability to reach and motivate the vast unserved hearing
impaired population (Kochkin, 2000). Therefore, each level of the Likert-like scale of this
study demonstrates graduated levels of adaptation based upon its intrinsic contribution
to failure to fit. Repeated remakes and modifications discourage patients and wears out
94
professionals. Returns for credit raise the cost of the next hearing aid sold and wear
down a delivery system that can ill afford the waste and loss. Levels 1 and 2 (i.e.,
comprised mostly with RFCs and remakes) represent the vast majority of the waste in
the industry. Level 3 (i.e., remakes that result in successful fitting outcomes) may be
necessary and mostly unavoidable, but seem to be closely tethered to dispenser
impression-taking skills (Pirzanski and Berge, 2002). Level 4 (i.e., significant in-office
modifications due to complaints of discomfort), while time-consuming, are much less
costly to the system and represent the nurturing process involved in auditory
rehabilitation. Level 5 (successful outcomes without the foregoing costs) allows
professionals to focus on the counseling and rehabilitative principles needed for the
happiest outcomes in meeting consumer expectations. Again, these are relatively
straightforward increments and provide for consistency in replication.
Another factor that may have affected internal validity was the state mandated
30-day trial period, which often fosters unrealistic consumer expectations and
encourages hearing health professionals to artificially speed up a process that should
otherwise require 60-90 days. About half of the states in the U.S. are already faced with
such a barrier to good practice (Federal Trade Commission, 2006). Such detrimental
regulations can adversely affect the outcomes of such investigations. Just as
importantly were issues of consistency in patient file notes and dispensing
professionals’ ability to elicit accurate reports from participants while quantifying that
which is usually considered qualitative: Cost-benefit, user compliance, and hidden
factors not addressed in this study (i.e., psychosocial factors and auditory limitations).
95
One measure that was felt should have been more sensitive in showing a more
direct relationship between variables was the limitation of only 3 levels of keratin status.
Perhaps four or five levels would have presented a better scatterplot line of best fit and r
score. However, while this might have helped internal validity and strengthen the test of
the hypothesis, creating 4 or 5 levels of differentiation of keratin would likely have
confounded external validity. For, even if our staff had achieved such a fine level
judgment, it is doubtful that the minutiae for that level of differentiation could reliably be
replicated by others without extensive training and precise duplication of equipment
settings.
Several factors in this study and its design affected external validity and, by
extension, replication in other practice settings. While the results indicate the sample
size to be sufficient, we did not analyze data on demographic make-up. Approximately
60% of participants in this study had Hispanic surnames, which could have introduced
some genetic influences into the EAC keratin status levels and other factors involved
with adaptation to prostheses. For instance, diabetes mellitus II (DMII), which could
adversely affect keratin formation and migration, is generally higher in this population
than for Caucasians (Boney, 2003; Mokdad, Ford & Bowman, 2000). Higher incidence
of DMII is known to affect sensitivity to earmold materials, cause more EAC
complications of fungal and pseudomonas otitis externa, neuropathy (Doyle & Hoffman,
1981).
The fact that this study contained a higher than usual ethnic population might
limit replication in other populations (Kricos, 2007; Fausti, 2003). However, this
investigator’s experience during frequent consultation trips throughout the nation has
96
found that there are usually off-setting conditions prevalent in other populations. These
prevalencies tend to yield roughly similar outcomes relative to keratin status. For
instance, older populations in more medically-intensive regions may be taking more
prescription medications than those in the rural, small town practice where the present
study was conducted. In other instances, public water supplies may influence more or
less mandibular or general osseous issues that can have a bearing upon neural firing of
mechanoreceptors, thereby affecting neuroreflex hypersensitivity and/or earmold
retention issues. Yet other regional differences may involve cultural, ethnic, dietary,
and/or levels of physical activity issues, which may have direct or indirect influence
upon ear homeostasis (Hoster, Melnick & Baker, 2002; Willott, 1991).
As mentioned in the limitations section of Chapter 3, a standard practice used by
DigiCare and all of its associate practices is the recommendation of a natural botanical
solution for all new hearing aid users. This would affect the generalization of outcomes
from this study unless all future studies utilized the same practice. Indeed, more than
3,000 dispensing and audiology practices in North America recommend the use of this
solution in a similar manner (Gad, 2006). In a previous observation study of 960 hearing
aid patients by DigiCare associate practices, dramatic results as a result of using
MiraCelltm botanical solution were seen in terms of mitigating scar tissues, calcium
plaques, stress cracks, unresolved adhesive otitis residue, minute perforations, and
other subclinical conditions of the TM (Chartrand, 2002). The most important benefit
appeared to be inspiration of normal EAC keratin growth. For increasingly, hearing
instrument practices see many first time patients with thinned or disturbed keratin as a
result of using cotton swabs (e.g., self-cleaning of the EAC), use of hydrogen peroxide
97
or other caustic solutions. In many cases, keratin is absent or peeled because of
chronic semi-dehydration, medication side-effects (i.e., certain diuretics), lifestyle
factors, untreated/improperly treated diabetes mellitus II, or other underlying disease
processes. It has been speculated by our staff that these increasing trends must be
having a deleterious effect upon the hearing aid fitting process everywhere. Therefore,
before the start of this study, we had already began standardizing use of this solution as
part of our best practice standards. This recommendation was made specifically for new
user preparation for wearing hearing aids.
For participants of the present study, MiraCelltm was recommended as a
preparatory protocol for the new hearing aid fitting. This investigator debated whether
use of the solution would produce outcomes that would skew the results of the study.
However, it was concluded that to not recommend the solution would be tantamount to
providing substandard care and might constitute a breach of ethics. Consequently, it
was recommended that they:
completely immerse their TM with the solution
place a wad of tissue at the entrance of the EAC to hold the solution in the ear
after about 15 minutes, they may remove the wad of tissue
The above procedure was to be done on a daily basis for a period of 10-14 days.
It was observed that this practice tended to raise the keratin status of many of those
with absent keratin and seemed to have little effect on those whose keratin was already
at level 2 or 3. It was noted that in some cases, participants were non-compliant or only
partially compliant with this recommendation. Some of the noncompliant participants
comprised most of the remake and RFC groups. Other noncompliant participants
98
required extensive and repeated in-office modifications to make their hearing
aids/earmolds comfortable, especially where overly sensitive neuroreflexes were
encountered, such as ear-cough (vagus/Arnold’s), hypervascularization of the TM
(trigeminal), and tissue swelling (lymphatic). Ostensibly because of the use of
Miracelltm, or at least the cessation of contributive factors, some of the level 1 (keratin)
participants ultimately were successful at adaptation to their hearing aids or earmolds.
Furthermore, it is assumed that some of the level 2 participants adapted better because
of use of the solution as a routine, concomitant protocol in the hearing aid fitting process
(Chartrand, 2002).
Implications and Need for Future Research
Certainly future investigators will be able to choose from more time-tested
instruments of measure and commonly accepted standards for differentiating keratin
and physiological variables than was enjoyed in this study. Perhaps some in the
hearing aid industry have already decided that these considerations are no longer an
issue with the advent of open-ear Over-the-Ear (OTE) fittings. After all, the benefits of
OTE fittings are--at this writing, at least--taking the industry by the proverbial storm.
Now accounting for more than 30% of all hearing aid fittings and growing, it would
appear that the problems covered in this paper are now of less importance to the
industry. For it is said of OTE fittings that: 1) there are no more occlusion and discomfort
issues with which to contend, 2) natural ear canal resonances are not being disturbed,
and 3) own-voice issues are decidedly resolved (Yanz & Olson, 2006; Schweitzer &
Jessee, 2006). Indeed, our clinic’s initial experiences with open-ear OTE fittings bear
out much of these observations. Fewer remakes and RFCs appear at this point to be an
99
important benefit of this new fitting configuration. However, more recently in trials in our
facilities in southern Colorado we have found that:
high frequency target gain is not being met in about 50% of such cases
that some comfort issues, especially dealing with itching of the EAC, still exist
because tubing and an expandable aerated tip still makes contact with the
mechanoreceptors of the EAC
that a more occluded, customized coupler would vastly extend the real ear
benefits of the new thin-tube technology
the primary target audience of this new configuration are those who have
previously eschewed amplification, mostly the mild and mild-to-moderate cases
that generally do not consider themselves candidates (this is a good outcome,
however, because it helps promote amplification in an otherwise intransigent
segment of the population)
the open-ear configuration will likely render successful in approximately 2535% of traditional hearing aid users, depending upon how many mild and mild
to moderate loss cases are added to the mix
Rose (2006) concurs with our findings and makes a powerful point in suggesting
that the current craze over open-ear BTE fittings is ignoring the dynamics of optimally
adapting amplification to the needs of patients who do not fit the narrowly defined profile
is considered ideal for such fittings. He foresees increased interest in (minimum contact)
custom earmolds coupled to the new OTE technology, which should of course refocus
the industry’s attention back to the need to observe and work with physiological
100
behavior of the ear under amplification. He proposes that to help this technology live up
to its promise, the following in part:
to expand the variety and availability coupling apparatus (tubing, tips, and
types of minimal contact earmold couplers
to more vigorously explore the directional (and spatial) issues that present in
open-ear configuration (non-existent under current paradigms)
to more objectively address the issue of increased acoustic feedback (and its
precursor, resonant distortion)
to address lingering comfort issues, such as EAC itching and contact
discomfort.
Our earmold lab at DigiCare Hearing Research & Rehabilitation has been
experimenting with minimum contact couplers that provide an addition 10-20dB more
gain without acoustic feedback or resonant distortion, but which still use the highfrequency-limiting thin tube. We note others’ efforts in producing receiver-in-the-canal
(RITE), but also see neuroreflex and physiological behavior issues not being addressed.
Our lab is also working to bring to market a new tubing that has an inside diameter (ID)
between the current choices of 0.70” (the inside diameter for standard tubing) and 0.35”
(inside diameter of OTE thin-tubing), perhaps settling for a medium-thin tubing that is
0.50” ID. While this may seem irrelevant to our discussion here, it may actually prove to
be the linchpin that can join minimum contact earmold technology to miniaturized OTE
digital technology. Thus, more hearing impaired individuals with serious losses may
enjoy the molded, thinner tube movement without sacrificing acoustical benefits,
perhaps closer to the 50% potential that has been estimated by some industry analysts
101
(Strom, 2006). For the other 50% of users (those with moderately severe to severe
losses), however, the issues of comfort and adaptability remain of immense importance.
Perhaps some of the built-in limitations for this huge group can be somewhat mollified
by making the larger behind-the-ear (BTE) devices and their respective acoustic
couplers more cosmetically appealing and ergonomically fitting. Hence, in this
investigator’s opinion, the trend away from custom instruments is probably a good one
from the physiological as well acoustic standpoint.
More important to our discussion are what kinds of research will be needed to
take the findings of this study to the next level. There are several:
In the pharmacological domain, there needs to be more research establishing
the benefits of commercially available botanical and other solutions, so that
beneficial remedies for the EAC can be accepted in the mainstream of medical
and clinical practice. The current research paradigm established by the FDA
discourages such research, because there are no patentable, multi-billiondollar fortunes to be made in such gentle, relatively benign solutions. Nor are
there any risks even approaching their more dangerous cousins in
otopharmacopia. Furthermore, we need to more candidly identify prescription
medications that cause loss of EAC keratin and which disturb the homeostasis
of the EAC. At this point, medications which cause dehydration, of which more
than 77 have been found to-date, head the list (Wrong Diagnosis, 2007; Facts
and Comparisons, 2005). This would include a host of hypertension, allergy,
asthmatic, and anti-inflammatory medications (see Table 1 for more examples).
In the dermatitis category come medications with adverse epithelial
102
implications. Anti-reflux medications bring loss of myelin sheath and pH
disturbances. All these and more can exacerbate successful adaptation to
hearing aids and earmolds, not to mention other (more serious) effects on
physiological and psychological behavior. Just as it required more than three
decades of research to finally bring ototoxicity of amino glycosides to
recognition in medical practice, we need to see the other oto-related aspects
explored, identified, and considered.
Better acoustic couplers and materials are needed to overcome comfort and
adaptation issues for a larger proportion of hearing aid users. This would
include the need for better impression-taking methodologies that capture the
dynamics of the living ear canal, not the static model now recognized. The ear
is designed to keep out anything foreign. Even the new soft materials are
relatively hard compared to the cellular properties of EAC tissues. But softer
materials mean more chemically active (and allergic) materials. So, the search
must be for medical grade silicones or other materials that do not introduce
pressure in mandible movement.
Most relevant to our discussion in this regard is the need for further research
into the mechanoreceptors and neuroreflexes of the EAC. Understanding these
will help us better understand how to work with the EAC’s immunological and
defensive mechanisms, rather than ignoring them as is the current practice.
There are at least two other neuroreflexes that we bumped into during our
research of the three included in this paper. One involves the tensor tympani
reflex relative to sound pressure levels of amplification and the damping at the
103
TM when higher levels of amplification are presented. The other involves ownvoice biofeedback reduction when pressure was applied to the Arnold’s Branch
of the Vagus at various points in the EAC. At this point, the industry tends to
lump these mysterious artifacts of amplification into the trash basket of acoustic
occlusion. However, any amount of venting fails to resolve these anomalies in
far too many individuals, because of unexplored tactile implications in the reflex
arc.
From a rehabilitative view, more persuasive data is needed to convince
regulatory agencies and consumer entities that there is a better way of
assuring efficacy and good business practice than the current reliance upon
the misguided 30-day trial. These hamper both hearing impaired individuals
and the industry with unnecessary failure to fits.
Conclusion
A staff of 17 professionals and technicians, and 98 hearing impaired participants
over a period of about three and a half years were involved in this study. The sample
population was reminiscent of that found in everyday private practice. It was proposed
that there was a strong correlative—though, not necessarily causal—relationship
between the status or thickness of external auditory canal keratin and participants’
ability to adapt successfully to the fitting of appropriate hearing aids and earmolds that
met their auditory and communicative needs. Benchmarks that helped identify
adaptation success were the rate of earmold/earshell remakes, in-office modifications,
and return for credit instruments. The frequency or absence of these represented the
degree of success in each hearing aid fitting.
104
Consequently, it was found that a strong relationship between the predictor
variable of keratin and the criterion variable of adaptation did exist. Furthermore, it was
found that the sub-criterion variables of remakes and returns for credit negatively
correlated with keratin status, so that as keratin status diminished the rate of remakes
and returns for credit increased. It is hopeful that the conclusions of this study will shine
a light onto a most important area of interest that heretofore been ubiquitously lacking
throughout the hearing aid industry, that of physiological behavior of the human external
auditory canal’s response to amplification. Moreover, it is hoped that these findings will
help promote improved designs, materials, and counseling modalities to help each and
every hearing impaired individual become a successful user of current hearing
technologies.
.
105
REFERENCES
Abrahams, D. (2005). True experimental designs and their meaning. True Experimental
Designs, retrieved on February 17, 2005, from
http://www.socialresearchmethods.net/ tutorial/Abrahams/true.htm.
American National Standards Institute (1996). ANSI standards for calibrating
audiometers. ANSI S3.6-1996.
Amin, M.R., and Koufman, J.A. (2001, July-August). Vagal Neuropathy after Upper
Respiratory Infection: A Viral Etiology? American Journal of Otolaryngology,
22(4):251-256.
Anonymousa (2003, May 13). Pathology; Pneumocytis carinii infection of ear presents
as indication of HIV. Retrieved on March 2, 2006, from
http://proquest.umi.com/pqdweb?index=65&did=334063301&SrchMode=1&sid=8
&Fmt
Anonymousb (2004, March 7). Ear Canal Cancer; Parotid may be invaded by squamous
cell external auditory canal carcinoma. Medication Devices & Surgical
Technology Week, p. 61.
APHAB (2006). Abbreviated Profile of Hearing Aid Benefit. University of Memphis,
School of Audiology and Speech-Language Pathology. Retrieved on May 2,
2006, from http://www.ausp.memphis.edu/harl/aphab.html.
Audiology and Hearing Aid Providers Registration, Rules and Regulation, (2002,
September 25). Colorado Department of Regulatory Agencies (DORA).
Retrieved on May 10, 2004, from http://www.dora.state.co.us/registrations/.
106
Bailey, D.M., and Schwartzberg, S.L. (1995). Ethical and Legal Dilemmas in
Occupational Therapy. Philadelphia: F.A. Davis Company, ISBN 0-8036-0567-6.
Barber, S. (2005, May 16). Hearing Aid “Sticker Shock” and Things to Consider When
Purchasing. Health Hearing, retrieved on January 24, 2006, from
http://www.healthyhearing.com/library/article_content.asp?article_id=710.
Barnett, E., and Klein, J.O. (1995). The problem of resistant bacteria for the
management of acute otitis media. Pediatric Clinics of North America 42: 509517.
Bell, J. (2006). Why Purchasing a Hearing Aid is a Consumer Nightmare. Retrieved on
May 10, 2006, from http://www.bio.net/bionet/mm/audiolog/1997September/003548.html.
Bensmaia, S.J. (2003). The vibrations of texture. The University of North Carolina at
Chapel Hill, AAT, (96 pages).
Bentler, R. (2000, October 31). Accepting New Truths in Hearing Aids. Healthy Hearing.
Retrieved on May 9, 2006, from http://www.healthyhearing.com/
library/article_content.asp?article_id=60.
Bento, R. (2006, January). Surgical management of intracranial complications of
otogenic infection. Ear, Nose, & Throat Journal. Retrieved on March 5, 2006,
from http://findarticles.com/p/articles/mi_m0BUM/is_1_85/ai_n16069063/.
Berke, J. (2006). Your Guide to Deafness/Hard of Hearing. Retrieved on May 9, 2006,
on http://deafness.about.com/mbody.htm.
Birsch, E.M., Logemann, J., Rademaker, A.W., Kahrilas, P.J, and Lazarus, C.L. (1994).
Pharyngeal effects of bolus volume, viscosity, and temperature in patients with
107
dysphagia resulting from neurologic impairment and in normal subjects. Journal
of Speech and Hearing Research, 37: 1041-1049.
Bloustine, S., Langston, L., and Miller, T. (1976, May-June). Ear-cough (Arnold’s) reflex.
Annals of Otorhinolaryngology, 85(3 pt 1): 406-407.
Boney, C. (2003, April 3). Type II diabetes in children and adolescents: An emerging
problem. Medicine and Health Rhode Island. Retrieved on January 12, 2007,
from http://findarticles.com/p/articles/mi_qa4100/is_200304/ai_n9205233.
Bordens, K.S., and Abbott, B.B. (2002). Research Design and Methods: A Process
Approach. San Francisco: McGraw-Hill ISBN 0-767-42152-3.
Boys Town (2006). Areas of Research—Clinical and Behavioral Studies of Human
Communication. Retrieved on May 4, 2006, from
http://www.boystownhospital.org/Research/areas/ClinicalBehavioral/hearing_aids
.asp.
Bray, V., Johns, M., and Ghent, R. (2001). Description and rationale of a digital, instant
fit hearing aid. Hearing Journal, 54(4): 38-46.
Briggs, R.D., and Gadre, A.K. (2001, November 7). Ototoxicity. Grand Rounds
Presentation, UTMB, Department of Otolaryngology.
Carlson, N.R. (2004). Physiology of Behavior (8th edition). Boston, MA: Allyn & Bacon.
ISBN 0-205-30840-6.
Chakeres, D.W., Kapila, A., and LaMasters, D (1985). Soft-tissue abnormalities of the
external auditory canal: subject review of CT findings. Radiology, 156 :105 –109.
Chartrand, M.S. (2007). How Much Water is Needed for Good Health? Colorado City,
CO: DigiCare Hearing Research & Rehabilitation, www.digicare.org.
108
Chartrand, M.S. (2006). Utilization of Neurophysiology of the External Auditory Canal in
Resolving Problem Hearing Aid Fittings. International Institute for Hearing
Instruments Studies, Livonia, MI.
Chartrand, M.S. (2004a, January-February). Ethical Dilemmas in Dispensing Practice.
The Hearing Professional.
Chartrand, M.S. (2005, May). Identifying “Neuroreflexes” of the External Ear Canal.
Audiology Online, retrieved on November 29, 2005 at
http://www.audiologyonline.com/articles/ arc_disp.asp? article_id=1374.
Chartrand, M.S. (2005, May). Effectiveness of Hearing Aid Manipulation Training for
Very Elderly Hearing Aid Users. Audiology Online. Retrieved on January 29,
2006, from www.audiologyonline.com/archives.
Chartrand, M.S. (2004, July-August). The Importance of the Keration Layer in
Successful Fitting and Treatment. The Hearing Professional, pp. 14-18.
Chartrand, M.S. (2003a, February). But What’s Wrong with Q-Tips? Audiology Online.
Retrieved on May 12, 2006, from http://www.audiologyonline.com/articles/
article_detail.asp?article_id=407.
Chartrand, M.S. (2003b). Video Otoscopy Observation and Referral: The FDA Red
Flags. The Hearing Professional, January-February, pp: 9-14.
Chartrand, M.S. (2003c). Diabetes Mellitus and Hearing Health 2003. Audiology Online,
Archives. Retrieved on May 17, 2006, from http://www.audiologyonline.com/
articles/article_detail.asp?article_id=458.
109
Chartrand, M.S. (2002). Video otoscopy observations of 960 patients using Miracell
botanical solution. Retrieved on December 3, 2006, from http://www.digicare.org/
Miracell.asp.
Chartrand, M.S. (1999). Hearing Instrument Counseling: Practical Applications in
Counseling the Hearing Impaired, 2nd edition. Livonia, MI: International Institute
for Hearing Instruments Studies.
Chartrand, M.S., and Chartrand, G.A. (2006, March). Resolving a Root Cause of Failure
to Fit: Re-examining the Ear Canal. The Hearing Review.
Chartrand, M.S., and Chartrand, G.A. (2004b). If A Tree Fell in the Forest: What’s
Really Holding Back the Market? The Hearing Review, 10(10): 21-23.
Checkosky, C.M. (1991). Temporal summation in the Pacinian channel: Implications for
the neural code for detecting vibrotactile stimuli. The University of Rochester,
AAT 9226009 (168 pages).
Columbia University (2006). Implantable Hearing Aids. Department of
Otolaryngology/Head and Neck Surgery. Retrieved on May 4, 2006, from
http://www.entcolumbia.org/implha.htm.
Corey, G., Corey, M.S., and Callahan, P. (2003). Issues and Ethics in the Helping
Professions, 6th edition. Pacific Grove, CA: Brooks-Cole, ISBN 0-534-35615-X.
Cox, R.M., Alexander, G.C., and Beyer, C.M. (2003, October). Norms for the
International Outcome Inventory for Hearing Aids. Journal of the American
Academy of Audiology, 14(8): 403-413.
Cox, R. M., and Alexander, G.C. (1999). Measuring satisfaction with amplification in
daily life: The SADL Scale. Ear and Hearing, 20: 306-320.
110
Cox, R.M., and Rivera, I.M., (1992). Predictability and Reliability of Hearing Aid Benefit
Measurement Using PHAB. Journal of the American Academy of Audiology, 3:
242-254.
Crandall, C.C., Kricos, P., and King, W. (1997, September 22-24). Long Term
Consequences of Hearing Aid Utilization. Conference Reports. National Institutes
of Health, Bethesda, Maryland.
Curtin, H.D., Wolfe, P., and May, M. (1982). Malignant external otitis: CT evaluation.
Radiology, 145: 383 –388
Davis, W. E. (1987). The external and middle ear, in J. Templar and W.E. Dais,
Otolaryngology—Head and Neck Surgery: Principles and Concepts. St. Louis,
MIssouri: Ishiyaku EuroAmerica, Inc., pp. 34—36.
Dash, G.I., and Kimmelman, C.P. (1988). Head and neck manifestations of sarcoidosis.
Laryngoscope, 98:50-53.
DigiCare (2006). Annual Report to the Board of Directors. DigiCare Hearing Research
& Rehabilitation, P.O. Box 19610, Colorado City, CO 81019, January 16, 2006.
DigiCare (2005). Hearing Aid Wearing Schedule. DigiCare Hearing Research and
Rehabilitation, Colorado City, Colorado.
DigiCare (2003). Notice of Privacy Practices. DigiCare Hearing Research &
Rehabilitation, P.O. Box 19610, Colorado City, Colorado 81019.
DiMartino, E., Walther, L.E., and Westhofen, M. (2005, November). Endoscopic
examination of the Eustachian tube: a step-by-step approach. Otolaryngological
Neurotology, 26(6): 1112-1117.
111
Doyle, N.D., and Hoffman, J. E., (1987). General Medical Considerations in Audiology,
in J. Katz, ed., Handbook of Clinical Audiology, 3rd edition, Baltimore: Williams &
Wilkins, pp. 39-47.
Edmunds, A.L. (2006, September 6). Ototoxicty. eMedicine from WebMD. Retrieved on
January 15, 2007, from http://www.emedicine.com/ENT/topic/topic699.htm.
Entlink (2001). Adam Politzer. Retrieved on December 23, 2005, from
http://entlink.net/museum/politzer.cfm? renderforprint=1.
Fausti, S. (2003). Epidemiology of Hearing Loss in Diabetic and Non-Diabetic Veterans.
VA Medical Center, Portland, OR.
Facts and Comparisons (2005). Drug Facts and Comparisons 2006, 60th edition. St.
Louis, MO: Wolters Kluwer Health, ISBN 1-57439-245-X.
Federal Register (1977, February 15). U.S. Food and Drug Administration Rules and
Reguations Regarding Hearing Aid Devices: Professional and Patient Labeling
and Conditions for Sale, Part IV. Federal Registers 9286-9296.
Federal Trade Commission (2006). The 30-day Hearing Aid Trial. Retrieved on
December 22, 2006, from http://www.ftc.gov/bcp/conline/pubs/health/hearing.pdf.
Frampton, M. (2003, June 23). Identifying and managing external ear problems. Pulse
(Tonbridge): p. 64-67.
Frolich, K., Kleinsasser, N., Rasp, and Staudenmaier, R. (2005, May 18). NavigationAssisted Constrruction of an External Ear Canal Using an Autogenous Foreskin
Graft. Otorhinolarynogology, 67: 137-141.
112
Gadd, R. (2006). Interview with the author concerning the current usage rate of Miracell
botanical solution among new hearing aid users today.
http://www.miracell.com/earcare.html.
Gartecki, D.C., and Erler, S.F. (1996). Older Adult Performance on the Communication
Profile for the Hearing Impaired. Journal of Speech and Hearing Research, 5: 2534.
Gislason, S. (2005). Immune Mediators: Chemical Connection in Immune Networks.
Retrieved on January 10, 2006, from http://www.nutramed.com/immunology/
mediators.htm.
Goddard, T. (2006). The Senior Citizen Protection Manual. Retrieved on May 10, 2006,
from http://www.azag.gov/seniors/spm.html#14.
Great Plains (2006). Breaking the Cycle with Ear Infections. Retrieved on May 2, 2006,
from http://www.greatplainslaboratory.com/ear-infection.html.
Guclu, B., Bolanowski, S.J., Pawson, L. (2003, July-August). End-to-End linkage (EEL)
clustering algorithm: A study on the distributions of meissner corpuscles in the
skin. Journal of Computational Neuroscience, Boston, 15(1): 19-27.
Gupta, D., and Verma, S., and Vishwakarma, S.K. (1986). Anatomic basis of Arnold’s
ear-cough reflex. Surgical Radiological Anatomy, 8(4): 217-220.
Haar, G.T. (2003, October 24). The Importance of Ototoxicity. Retrieved on January 10,
2007, from http://www.vin.com/proceedings.plx?cid=wsava2003&pid
=6566&0=Ge.
Hadjikoutis, S., Wiles, C.M., and Eccles, R. (1999). Cough in motor neuron disease: A
review of Mechanisms. Quarterly Journal of Medicine, 92: 487-494.
113
Handzel, O., and Halperin, D. (2003, July 15). Nectrozing (malignant) external otitis.
American Family Physician, 68(2): 309-313.
Hawke, M. (1981). “Atlas of Clinical Otoscopy”, Modern Medicine of Canada, 36(3):
303-310.
Hawke, M., and Jahne, A.F. (1987). Diseases of the Ear—An atlas and integrated text.
Philadelphia, PA: Gower Press/Lea and Febiger.
Hearing Industries Association (2006, April). HIA Statistical Reporting Program: First
Quarter 2006. Alexandria, VA: HIA.
Herbst, K., and Humphrey, C. (1980). Hearing impairment and mental state of the
elderly living at home. British Medical Journal of Clinical Research, 281: 903-905.
Heilbrun, M.E., Salzman, C.M., Glastonbury, C.M., Harnsberger, H.R., Kennedy, R.J.,
and Shelton, C. (2003, April). External Auditory Canal Cholesteatoma: Clinical
and Imaging Spectrum. American Journal of Neuroradiology 24:751-756.
Hoover, B.M., Stelmachowicz, P.G., and Lewis, D.E. (2000). Effect of earmold fit on
predicted real ear SPL using a real ear coupler difference procedure. Ear and
Hearing, 21: 310-317.
Hoster, A.S., Melnick, T.A., and Baker, C.T. (2002, Spring). Prevalence of Activity
Limitation among Adults in New York State, 1993-1998. New York State Health
Department.
Humes, L.E., and Wilson, D.L. (2003, February). An examination of changes in hearingaid performance and benefit in the elderly over a 3-year period of hearing-aid
use. Journal of Speech, Language, and Hearing Research, 46: 137-145.
114
Huntley, A. (1995, December). Diabetes Mellitus: Review. Dermatology Online Journal,
1(2). Retrieved on November 5, 2005, from
http://dermatology.cdlib.org/DOJvol1num2/ diabetes/ dmreview.html.
IIHIS, (2006). A Practical Course in Neurophysiology and Video Otoscopy. Livonia, MI:
International Institute for Hearing Instruments Studies.
IIHIS, (2006). Licensing & Certification Exam Prep Course. Livonia, MI: International
Institute for Hearing Instruments Studies.
Infoscan Data (2003). Figure 8 Sales of Cotton Balls 1997-2002. Retrieved on January
26, 2006, from http://reports.mintel.com/sinatra/reports/index/
&letter=6/display/id=2&anchor=a2.
Jahne, A.F., and Cook, E.W. (1997). Medical Issues Related to CIC Fitting. In M.
Chasin, (Ed.), CIC Handbook (pp. 53-68).San Diego, CA: Singular Publishing
Group, Inc.
Jahne, A.F., and Hawke, M. (1983). Infections of the external ear. In C. Cummings. J.
Fredrickson, L. Harker, C., C. Crause, and D. Schuller (Eds.). Otolaryngology—
Head and Neck Surgery (2nd ed., pp. 2787-2794). St. Louis, MO Mosby-Year
Book, Inc.
Jahnke, V. (1976). Morphology and clinical significance of syringomas of the external
auditory canal. Larygological Rhinological Otology (Stuttg), 55(3): 210-216.
Jegoux, F., Legent, F., and Beauvillain de Montreuuil, C (2002, August 24). Chronic
cough and ear wax. The Lancet, London, 360(9333): 618-620.
115
Jenstad, L.M., Van Tassell, D.J., and Ewert, C. (2003, September). Hearing Aid
Troubleshooting Based on Patients’ Descriptions. Journal of the American
Academy of Audiology, 14(7): 347-360.
Johnson, A., and Hawke, M. (1988). “The nonauditory physiology of the external ear.”
From Physiology of the Ear, J. Santos-Sacchi, ed, New York: Raven Press, pp.
41-58.
Kavanaugh, K. (2007). Ear Canal Photographs. ENTUSA, retrieved on January 15,
2007, from http://www.entusa.com/external_ear_canal.htm.
Kemp, R. J., and Bankaitis, A. E. (2000). Infection control for audiologists, in HosfordDunn H., Roeser, R., and Valente, M., eds. Audiology Diagnosis, Treatment, and
Practice Management, Vol. III, New York: Theime Publishing Group.
Kennedy, M. and Volz, P. (1983). Dissemination of yeasts after gastrointestinal
inoculation in antibiotic-treated mice. Sabouradia 21:27-33.
Kinsman, O.S., and Pitblado, K. (1989). Candida albicans gastrointestinal colonization
and invasion in the mouse: Effect of antibacterial dosing, antifungal therapy, and
immunosuppression. Mycoses 32:664-74.
Kochkin, S. (1999). Reducing hearing instrument returns with consumer education. The
Hearing Review, 6(10): 18-20.
Kochkin, S. (2000). Why my hearing aids are in the drawer: The consumer’s
perspective. Hearing Journal, 52(2): 39-41.
Kress, M., and Zeilhofer, H.U. (1999). Capsaicin, protons, and heat: New excitement
about nociceptors. Trends in Pharmacological Science, 20:112-118.
116
Kricos, P.B. (2006, January 16). Minimizing the Effects of Non-Audiological variables on
Hearing Aid Outcomes. Audiology Online, retrieve on January 16, 2007, from
www.audiologyonline.com.
Kryzanski, W. (1997). Analysis of a model of membrane potential for a skin receptor
nerve. State University of New York at Buffalo, AAT 9801317, (150 pages).
Liston, S.L., and Siegel, L.G. (1986). Tinactin in the treatment of fungal otitis externa.
Laryngoscope, 96: 699.
Luettig, B. (1984). Macrophage activation and induction of macrophage cytotoxicity by
purified polysaccharide fractions from the plant Echinacea purpurea. Infection
Immunity 46:845-849.
Magelin, G. (2004, February 4). Comments Opposing the Etymotic and Gudhear
Petitions Docket Numbers 2003P-0362 and 2003P-0363. Testimony before the
Division of Dockets, Food and Drug Administration, Rockville, MD.
Mann, R.J., and Peaehcy, R.D. (1983). Sarcoidal tissue reaction another complication
of ear piercing. Clinical & Experimental Dermatology, 8:199-200.
Matsunaga, T., and Hirota, E. (2002, September 18). Familial lateral canal malformation
with external and middle ear abnormalities. American Journal of Medical
Genetics Part A, 116A(4): 360-367.
Mbao, M.N., Eikelboom, R.H., Atlas, M. D., Gallop, M.A. (2003, December). Evaluation
of Video-Otoscopes Suitable for Tele-Otology. Telemedicine Journal and eHealth, 9(4): 325 -330.
117
McSpaden, J. B., and McSpaden-Hickock, D. K., and Hickock, R. L. (1993, JanuaryFebruary). More than everything you wanted to know about non-acoustic
occlusion and venting. Audecibel.
Merck (2006). Function of Biotin. Retrieved on May 15, 2006, from
http://www.merck.de/servlet/PB/menu/1284910_ePRJ-Merck-PigmenteCOSMETIC.
Mims-Voll, L., and Jones, C.H. (1998). CICs: Five Years Later, What Have We
Learned? The Hearing Review, 5(4): 8-12.
Mokdad, A., and Ford, E., and Bowman, B. (2000). Diabetes Trends in the United
States: 1990-1998. Diabetes Care, 23:1278-1283.
Molina, M.A., Gamboa, E.M., Tello, P.C., Benavides, P.Z., Leon, L.C., Guerra, R.T., and
Padilla, C.R. (2006, Spring). Spontaneous inflammatory cytokine gene
expression in normal human peripheral blood mononuclear cells. Lymphatic
Biology Research, 4(1): 34-40.
Nafe, J.P., and Wagoner, K.S. (1941). The nature of pressure adaptation. Journal of
General Psychology, 25: 323-351.
Naiberg, J., Berger, G., and Hawke, M. (1984, October). The pathologic features of
keratosis obturans and cholesteatoma of the external auditory canal. Archives of
Otolaryngology—Head & Neck Surgery, 110(10): 751-756.
NIDCD (2006). Hearing Aids. National Institute on Deafness and Other Communication
Disorders. Retrieved on May 4, 2006, from
http://www.nidcd.nih.gov/health/hearing/hearingaid.asp.
118
Nordin, M. (1990). The innervation of the human face studies based on
microneurographic recordings. Uppsala Universitet (Sweden), AAT C221189.
Oliviera, R., Babcock, M., Venem, M., Hoeker, G., Parish, B., and Vasant, K. (2005,
February). The Dynamic Ear Canal and its Implications: The problem may be the
ear, and not the impression. The Hearing Review, 12(2):18-19, 82.
Oliviera, R., Hammer, B., and Stillman, A. (1992). MRI of the external auditory canal.
Audiology Today, 4(2): 48.
Oliviera, R., Hammer, B., Stillman, A., Jons, C., Margolis, R., and Holm, J. (1992). A
look at ear canal changes with jaw motion. Ear & Hearing, 13(6): 464-466.
Ostfeld, E. , Rubinstein, E., Gazit, E., and Smetana, Z. (1977). Effect of systemic
antibiotics on the microbial flora of the external ear canal in hospitalized children.
Pediatrics 60: 364-66.
Pare, M., Elde, R., Mazurkiewics, J.E., Smith, A.M., and Rice, F.L. (2001, September).
The meissner corpuscle revised: A multiafferented mechanoreceptor with
nociceptor immunochemical properties. Journal of Neuroscience, 21(18): 72367246.
Park, K., Chun, Y.M., Park, H.J., and Lee, Y.D. (1999). Immunohistochemical study of
cell proliferation using BrdU labeling on tympanic membrane, external auditory
canal and induced cholesteatoma in Mongolian gerbils. Acta Otolaryngol, 119:
874 –879.
Persaud, R.A.P, Hajioff, D., Thevasagayam, M.S., Wareing, M.J., and Wright, A. (2004,
December). Keratosis obturans and external ear canal cholesteatoma: How and
119
why we should distinguish between these conditions. Clinical Otolaryngology &
Allied Sciences, 29(6): 577-581.
Peterson, M. E., and Bell, T.S. (2004, January). Factors Influencing Hearing Aid Return
and Exchange Rates. The Hearing Review, 11(1). Retrieved on March 5, 2006,
from http://www.hearingreview.com/articles.asp?articleid=H0401F01.
Pirzanski, C., and Berge, B. (2002, March 18). Ear Impressions: Art or Science?
Audiology Online. Retrieved on December 5, 2005, from
http://www.audiologyonline.com/articles/pf_arc_dosp. asp?article_id=336.
Prairie, J. (2005). Rosacea & Skin Function: Treating Keratinocytes. Retrieved on
December 22, 2005, from http://rosacea.derm.net.au/rosacea-functioning/
keratinocytes.html.
Pursell, K.J., et al. (1992). Aspergillus species colonization and invasive disease in
patients with AIDS. Clinical Infectious Diseases, 14: 141.
Ramsdell, R.S. (1978). The psychology of the hard-of-hearing and the deafened adult.
In H. Davis & S.R. Silverman (Eds.), Hearing & Deafness. New York: Holt,
Rhinehart & Winton
Reid, C.C. (1922). Lymphatics of the Eye, Ear, Nose and Throat. In F.P. Millard, ed.,
Applied Anatomy of the Lymphatics (out of print), retrieved on February 13, 2005,
from http://www.meridianinstitute.com/eamt/files/millard/millcont.html.
Resnick, S.B. (1999). Breakdown in the fitting process; An idiopathic approach to
Fitting: A Matter of Perspective. Retrieved on November 9, 2005, from
http://www.vard.org/ mono/ ear/resnick.htm.
120
Robert, W., Funnell, J., and Laszlo, C.A. (2005, January 2). A critical review of
experimental observations on eardrum structure and function. McGill University,
Biomedical Engineering Unit and Department of Otolaryngology, retrieved on
November 1, 2005, from http://funsan.biomed.mcgill.ca/-funnell/AudiLab/ear.html.
Roland, P.S., and Meyerhof, W.L. (1998). Otosclerosis. Head and neck SurgeryOtolaryngology, II: 2083-2097.
Roland, P. S. (2001). Chronic External Otitis. Ear, Nose & Throat Journal, June .
Rose, D.E., (2006, August). The return of the earmold: For some open fittings, using a
custom earmold would result in a better fit. Hearing Review. Retrieved on
November 25, 2006, from http://www.hearingreview.com/
article.php?s=HR/2006/08&p=3.
Ross, M. (2005). Why People Won’t Wear Hearing Aids. Rehabilitation Engineering
Research Center on Hearing Enhancement. Retrieved on May 9, 2006, from
http://www.hearingresearch.org/Dr.Ross/why_people_wont_wear_hearing_aids.h
tm.
Ross, M. (2004, November 1). Redefining the hearing aid selection process. Audiology
Online. Retrieved on January 14, 2007, from
http://www.audiologyonline.com/articles/article_detail.asp?article_id=1211
Ross, M. (2001, November/December). Developments in Research and Technology.
The Journal of Self-Help for Hard of Hearing People. Retrieved on March 27,
2006, from http://www.hearingloss.org/html/ear_molds.html.
121
SADL (2006). Satisfaction with Amplification in Daily Life (SADL). University of
Memphis, School of Audiology and Speech-Language Pathology. Retreived on
May 2, 2006, from http://www.ausp.memphis.edu/harl/sadl.html.
Sahelian, R. (2006). Yeast Infection-Candida Infection. Retrieved on May 4, 2006, from
http://www.raysahelium.com/infection/html.
Samonis, G., Gikas, A., and Anaissie, E. (1993). Prospective evaluation of the impact
of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans.
Antimicrobial Agents and Chemotherapy 37: 51-53, 1993.
Sandeep, V. and Hayward, R.A. (2003, April). Treatment of Hypertension in Type 2
Diabetes Mellitus: Blood Pressure Goals, Choice of Agents, and Setting Priorities
in Diabetes Care. Annals of Internal Medicine, 138(7): 593-602.
Sandlin, R.E., ed. (1996). Hearing Instrument Science and Fitting Practices, 2nd edition.
Livonia, MI:International Institute for Hearing Instruments Studies, p. 905-932.
Schoppmann, S.F. (2005, November-December). Lymphangiogenesis, inflammation
and metastasis. Anticancer Research, 25(6C): 4503-4511.
Schot, L.J., Hilgers, F.J., Keus, Schouwenburg, P.F., Drechler, W.A. (1992, October).
Late effects of radiopathy on hearing. European Archives of Oto-RhinoLaryngology, 249(6): 305-308.
Schweitzer, C., and Jessee, S.K., (2006, September). The Value of open-fit hearing
aids. Hearing Review. Retrieved on November 25, 2006, from
http://www.hearingreview.com/article.php?s=HR/2006/09&p=3.
122
Skin Cancer Foundation (2006). Actinic Keratosis: What you should know about this
common precancer. Retrieved on January 19, 2006, from
http://www.skincancer.org/ak/index.php.
Slattery, W. H. III, and Saadat, P. (1997). Postinflammatory medial canal fibrosis.
American Journal of Otolaryngology, 18:294—297.
Smoliar, E, Smoliar, A., and Belkin, VS. (1999). Innervation of Human Trigeminal Nerve
Blood Vessels. Cells, Tissues, Organs, 165(1): 40-44.
Spyries, B. (2003, April 10). Bone-Anchored Hearing Aids. AuD Paper, Nova
Southeastern University.
Staab, W. (1990, Spring). Hearing in the Elderly, Volume II. Audecibel (Journal of the
International Hearing Aid Society).
Staab, W. (1997). Deep Canal Hearing Aids. In M. Chasin, (Ed.), CIC Handbook (pp. 130).San Diego, CA: Singular Publishing Group, Inc.
Stakeholder Forum (2001). Stakeholder Forum on Hearing Enhancement, Earmolds:
Problem Statement. RERC, retrieved on December 23, 2005, from
http://cosmos.buffalo.edu/t2rerc/pubs/forums/hearing/forum/earmolds/problem_st
atement.
Steuer, M.K., Hofstadter, F., Probster, L., Beuth, J., and Strutz, J., (1995, May-June).
Are ABH antigenic determinants on human outer ear canal epithelium
responsible for Pseudomonas aeruginosa infections? Journal of
Otorhinolaryngological Related Specialties, 57(3): 148-152.
Stewart, R.Hh., Quick, C.M., Zawieja, D.C., Cox, C.S., Allen, S.J., and Laine, G.A.,
(2006). Pulmonary air embolization inhibits lung lymph flow by increasing
123
lymphatic outflow pressure. Michael E. DeBakey Institute, Texas A&M University,
College Station.
Sweetow, R.W., Bratt, L.A., Miller, M., and Hendeson-Sabes, J., (2004, January). A
Time-Cost Analsys with Patients Whop Purchase, Return and Exchange Hearing
Aids. Hearing Review, 11(1).
Strom, K. (2000, June). Staff Standpoint. The Hearing Review. Retrieved on January
10, 2007, from http://www.hearingreview.com/issues/articles/2002-06_06.asp.
Strom, K. (2005, December). Dispenser Survey. The Hearing Review.
Sullivan, R.F. (1995, Novemeber-December). Video Otoscopy: Basic and Advanced
Systems. The Hearing Review, 2(10): 12-16.
Takahashi, M., Yoshimoto, T., and Kubo, H., (2004, July). Molecular mechanisms of
lymphangiogenesis. International Journal of Hematology, 80(1): 29-34.
Tille J.C., and Pepper, M.S., (2004, September). Hereditary vascular anomalies: New
insights into their pathogenesis. Arteriosclerosis, Thrmombosis, and Vascular
Biology, 24(9): 1578-1590.
Trace, R., (1996, March 4). Video Otoscopy: Applications in Audiology. Advance for
Speech-Language Pathologists & Audiologists, 6(9).
Van Boven, R.W., (2000, November). Tactile spatial resolution in blind Braille readers:
Reply. Neurology, 55(10): 1597.
Van der Gaag, I., (1986, October). The pathology of the external ear canal in dogs and
cats, Veterinary Quarterly, 8(4): 307-317.
124
Van Hecke, M.L., (1994). Emotional responses to hearing loss. In Effective counseling
in audiology: Perspectives and Practices, J.G. Clarke, and F.N. Martin, eds.
Englewood Cliffs, N.J.: Prentice-Hall, Inc., pp. 92-115.
Vincek, V. (2005, November). Primary carcinosarcoma of the helix of the ear. Ear,
Nose. Throat Journal. Retrieved on March 5, 2006, from
http://findarticles.com/p/articles/mi_m0BUM/is_11_84/ai_n15896771/print.
Vrabic, J., and Underbrink, M., (2001, March 21). Grand Rounds Presentation:
Infections of the External Ear. University of Texas, Department of
Otolaryngology/Head and Neck Surgery.
Watanabe, I.S., (2004, June) Ultrastructures of mechanoreceptors in the oral mucosa.
Anatomical Science International, 79(2): 55-61.
Waters, T.J., (2003, January 14). Legal Opinion re Colorado law for Audiologists and
Hearing Aid Providers. Rye, CO: Aural Rehab Concepts.
Wayner, D.S., (2005, December). Aural Rehabilitation adds value, lifts satisfaction, cuts
returns. The Hearing Journal, 58(12): 30-38.
Weigland, J., Bodkin, K., Madell, J.R., Rosenfeld, R.M., Press, I.L., (2002). By the
numbers: A statistical analysis of hearing instrument returns. The Hearing
Review, 9(1): 22-28, 49.
Willott, J. F. (1991). Aging and the Auditory System: Anatomy, Physiology and
Psychoacoustics. San Diego: Singular Publishing Group, pp. 82-88.
Winter, H., Schweizer, J., and Goerttler, K., (1983). Keratin polypeptide composition as
a biochemical tool for the discrimination of benign and malignant epithelial
lesions in man. Archives Dermatology Research, 275(1): 27-34.
125
Wrong Diagnosis, (2007). Medication Causes of Dehydration. Retrieved on January 14,
2007, at http://www.wrongdiagnosis.com/d/dehydration/medic.htm#drug_
interactions.
Yanz. J., and Olson, L. (2006, February). Open-Ear Fittings: An Entry into Hearing Care
for Mild Losses. The Hearing Review, 13(2): 48-52.
126
APPENDIX A
Participant Data Sheet
Participant Information
No. __________ Sex/Age__________ Reviewer_____________ Month/Year Fitted_______________
Fitting Information (check appropriate box)
Mild HL Moderate HL Severe HL
Monaural
Binaural
HF Loss
BTE
Reverse Slope Cookie Bite HL
ITE
CC
Analogue
Digital
Case Notes in File
Keratin Status (Check appropriate boxes):
Absent
Peeling
Barely Visible
Thin
Medium
Thick
Hearing Aid Adaptation (Check appropriate boxes):
Return of all of a monaural or binaural fitting
Return one side of a binaural fitting for reasons of discomfort
Exchange model because of adaptation issue
Remake shells/earmolds for all of a monaural or binaural fitting
Remake shell/earmold for one ear in a binaural fitting
Unable to wear all day by end of initial two weeks but OK afterwards
Significant in-office modification as a result of complaint
Complaints of discomfort in ear(s) without need for modification
Physical adaptation without reports of the above over initial 30 day period
Misc. Notes:__________________________________________________________
____________________________________________________________________
127
APPENDIX B
Keratin/Adaptation Rating Scale
Participant Information
No. __________ Sex/Age__________ Reviewer_____________ Month/Year Fitted_______________
Fitting Information (check appropriate box)
Monaural
Binaural
BTE
ITE
CC
Analogue
Keratin Rating (3 levels)
Peeling or Absent Keratin………………………………………………………… 1
Barely Visible or Thin Keratin…………………………………………………… 2
Medium or Thick Keratin………………………………………………………......3
Hearing Aid Adaptation Success Rating (5 levels)*
Return all instruments in a monaural or binaural fitting…………………….....1
Return one side of a binaural fitting for reasons of discomfort AND/OR
Exchange model because of adaptation issue AND/OR
Remake shells/earmolds for all of a monaural or binaural fitting….………….2
Remake shell/earmold for one ear in a binaural fitting AND/OR
Unable to wear all day by end of initial two weeks but OK afterwards…….…3
Significant in-office modification as a result of complaint AND/OR
Complaints of discomfort in ear(s) without need for modification………….….4
Physical adaptation without any of the above over initial 30-day period…..…5
*NOTE: IN CASE OF MULTIPLE RESPONSES, RATE AT LOWEST LEVEL
FINAL ADAPTATION RATING_________
Digital
128
APPENDIX C
HIPAA Informed Consent Form
In accordance with provisions required in the federal law titled Health Insurance
Portability and Accountability Act of 1996 (HIPAA), I hereby give my consent to
DigiCare® Hearing Research and Rehabilitation of Colorado to share necessary patient
information with entities that provide hearing instruments, earmolds, research,
consultation, or reimbursement on my behalf. I understand that all patient information
pertaining to my files will be held confidentially as provided by the Notice of Privacy
Practices posted at each DigiCare® location.
____________________________________________
Patient Signature
____________________________________________
Print Patient Name
________________________
Date
129
APPENDIX D
(DigiCare® Notice of Privacy Policy)
HEARING HEALTHCARE PROFESSIONAL AUTHORIZATION
By signing this form, I understand that I am giving DigiCare Hearing Research &
Rehabilitation authorization to use or disclose the following information beyond that
which is customary for treatment, referral, research, and other purposes already
covered in a previous authorization:
Specify information and to whom it may be disclosed:
______________________________________
Right to Revoke. I understand that I may restrict the individuals or organizations to
whom my healthcare information is released. Further, I understand that I may revoke
my authorization at any time; however, my revocation must be in writing, mailed to
DigiCare Hearing Research & Rehabilitation at the office address listed below, and
DigiCare Hearing Research & Rehabilitation must only comply with such revocation to
the extent it is consistent with its Notice of Privacy Practices.
Redisclosure. Information that DigiCare Hearing Research & Rehabilitation uses or
discloses based on the authorization I am giving may be subject to re-disclosure by the
130
person who receives the information and may no longer be protected by the federal
privacy rules.
Refusal. I have the right to refuse to give DigiCare Hearing Research & Rehabilitation
this authorization. If I do not give the authorization, it will not affect the treatment I
receive or the methods used to obtain reimbursement for my care, except, however, if
my treatment at DigiCare Hearing Research & Rehabilitation is for the sole purpose of
creating health information for disclosure to the recipient identified in this Authorization,
in which case, DigiCare Hearing Research & Rehabilitation may refuse to treat me if I
do not sign this Authorization.
Inspect/Copy. I may inspect or copy the information that DigiCare Hearing Research &
Rehabilitation may send at any time.
Term. This notice is effective as of the date set forth below and will remain in effect until:
{CHECK ONE OF THE FOLLOWING:}
____ The following date or
event:___________________________________________________
____ DigiCare Hearing Research & Rehabilitation fulfills the request.
____ I provide written notice of revocation to DigiCare Hearing Research &
Rehabilitation. The revocation will be effective immediately upon DigiCare Hearing
Research & Rehabilitation’s receipt of my written notice, except that the revocation will
131
not have any effect on any action taken by DigiCare Hearing Research & Rehabilitation
in reliance on this Authorization before it received my written notice of revocation.
Purpose. I authorize DigiCare Hearing Research & Rehabilitation to use or disclose my
health information in the manner described above to the recipient for the term for the
following specific purpose {“At the request of the patient” is sufficient if the patient is
initiating the Authorization:}
______________________________________________________________________
Contact. I may contact DigiCare Hearing Research & Rehabilitation’s privacy
officer by mail at: P.O. Box 19610, Colorado City, CO. 81019-9610 or by telephone
at (719) 676-3277.
Authorization. I have read and understand the terms of this Authorization, and
have received a copy for my records. I hereby authorize DigiCare Hearing
Research & Rehabilitation to use or disclose my health information in the manner
described above.
________________________________________
Signature of patient or personal representative
_______________________________________
Printed name of patient/personal representative
_________________________
Effective Date
__________________________
Personal Representative’s Authority
132
APPENDIX E
DATA FROM KERATIN/ADAPTATION RATING FORMS
Case#
001
002
003
004
005
006
007
008
009
010
011
012
013
014
015
016
017
018
019
020
021
022
023
024
025
026
027
028
029
030
031
032
033
034
035
036
037
038
039
040
041
042
043
Gender
1.00
2.00
1.00
2.00
1.00
2.00
1.00
1.00
2.00
2.00
2.00
1.00
2.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
2.00
2.00
1.00
2.00
2.00
2.00
1.00
2.00
1.00
1.00
2.00
1.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
Age Fitting
67.00 2.00
93.00 2.00
72.00 1.00
70.00 1.00
76.00 1.00
63.00 1.00
74.00 2.00
67.00 1.00
87.00 2.00
82.00 2.00
80.00 2.00
79.00 1.00
29.00 1.00
68.00 2.00
83.00 2.00
72.00 2.00
67.00 1.00
64.00 2.00
75.00 1.00
70.00 1.00
48.00 1.00
55.00 1.00
76.00 2.00
73.00 2.00
67.00 2.00
78.00 2.00
70.00 1.00
87.00 1.00
88.00 2.00
77.00 1.00
76.00 1.00
50.00 2.00
95.00 2.00
61.00 1.00
66.00 1.00
60.00 1.00
81.00 2.00
76.00 2.00
33.00 1.00
77.00 2.00
70.00 2.00
80.00 2.00
65.00 2.00
Type
1.00
2.00
2.00
2.00
2.00
1.00
1.00
2.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
2.00
1.00
2.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
2.00
1.00
1.00
2.00
2.00
1.00
1.00
2.00
1.00
2.00
1.00
Remake
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
2.00
1.00
1.00
1.00
2.00
2.00
2.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
RFC
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
Keratin Adaptation
2.00
2.00
3.00
5.00
3.00
5.00
2.00
5.00
3.00
5.00
1.00
3.00
2.00
4.00
1.00
5.00
3.00
5.00
2.00
4.00
3.00
5.00
3.00
5.00
2.00
4.00
1.00
4.00
2.00
2.00
2.00
4.00
2.00
3.00
2.00
5.00
3.00
5.00
3.00
5.00
1.00
2.00
2.00
5.00
1.00
1.00
2.00
4.00
1.00
3.00
2.00
4.00
2.00
5.00
2.00
5.00
1.00
1.00
2.00
5.00
3.00
5.00
1.00
3.00
1.00
1.00
3.00
5.00
3.00
5.00
3.00
5.00
3.00
5.00
2.00
4.00
2.00
4.00
1.00
4.00
1.00
4.00
1.00
3.00
2.00
5.00
133
044
045
046
047
048
049
050
051
052
053
054
055
056
057
058
059
060
061
062
063
064
065
066
067
068
069
070
071
072
073
074
075
076
077
078
079
080
081
082
083
084
085
086
087
088
089
090
091
092
093
094
2.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
2.00
1.00
1.00
2.00
2.00
2.00
1.00
1.00
2.00
1.00
1.00
1.00
2.00
1.00
1.00
2.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
2.00
1.00
42.00
85.00
54.00
69.00
68.00
67.00
60.00
59.00
67.00
64.00
71.00
75.00
78.00
68.00
71.00
72.00
71.00
44.00
39.00
88.00
61.00
87.00
85.00
84.00
79.00
67.00
63.00
66.00
89.00
67.00
72.00
78.00
62.00
54.00
77.00
67.00
61.00
58.00
63.00
64.00
83.00
70.00
76.00
87.00
85.00
74.00
81.00
61.00
88.00
67.00
74.00
1.00
2.00
2.00
2.00
1.00
2.00
2.00
2.00
2.00
2.00
2.00
1.00
2.00
1.00
2.00
1.00
2.00
2.00
1.00
2.00
1.00
2.00
2.00
1.00
1.00
2.00
1.00
2.00
2.00
2.00
1.00
2.00
1.00
1.00
2.00
1.00
2.00
2.00
1.00
2.00
2.00
1.00
2.00
2.00
2.00
1.00
2.00
2.00
2.00
1.00
1.00
1.00
1.00
2.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
2.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
2.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
3.00
3.00
3.00
1.00
3.00
2.00
3.00
3.00
2.00
1.00
3.00
3.00
2.00
3.00
2.00
2.00
2.00
2.00
2.00
3.00
1.00
2.00
2.00
2.00
3.00
2.00
2.00
1.00
2.00
2.00
3.00
3.00
3.00
2.00
3.00
3.00
3.00
3.00
1.00
2.00
3.00
3.00
2.00
3.00
2.00
3.00
2.00
3.00
3.00
3.00
3.00
5.00
5.00
5.00
3.00
5.00
2.00
5.00
5.00
5.00
4.00
4.00
5.00
4.00
4.00
3.00
4.00
5.00
4.00
5.00
5.00
2.00
3.00
5.00
4.00
5.00
2.00
4.00
3.00
5.00
4.00
5.00
4.00
5.00
4.00
5.00
5.00
4.00
4.00
1.00
5.00
4.00
5.00
3.00
5.00
5.00
5.00
4.00
4.00
4.00
4.00
5.00
134
095
096
097
098
1.00
1.00
2.00
2.00
56.00
52.00
94.00
78.00
2.00
1.00
2.00
1.00
1.00
1.00
1.00
2.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
1.00
3.00
2.00
2.00
4.00
5.00
3.00
5.00
135
APPENDIX F
SPSS(GP 14.0) Calculations
1. SPSS(GP) calculations for regression re keratin vs adaptation.
Regression
De scri ptive Statistics
Adaptation
Keratin
Mean
4.1224
2.2245
St d. Deviat ion
1.09606
.73961
N
98
98
Corre lations
Pearson Correlation
Sig. (1-tailed)
N
Adaptation
Keratin
Adaptation
Keratin
Adaptation
Keratin
Adaptation
1.000
.627
.
.000
98
98
Keratin
.627
1.000
.000
.
98
98
Variables Entered/Removedb
Model
1
Variables
Entered
Keratin a
Variables
Removed
.
Method
Enter
a. All requested variables entered.
b. Dependent Variable: Adaptation
Mode l Summary
Change Statistics
Model
1
R
R Square
.627a
.393
Adjusted
R Square
.387
a. Predictors: (Constant), Keratin
Std. Error of R Square
the Estimate Change
.85826
.393
F Change
62.200
df1
df2
1
96
Sig. F Change
.000
136
ANOV Ab
Model
1
Regres sion
Residual
Total
Sum of
Squares
45.817
70.714
116.531
df
1
96
97
Mean Square
45.817
.737
F
62.200
Sig.
.000a
a. Predic tors : (Const ant), Keratin
b. Dependent Variable: A daptation
Coefficientsa
Model
1
(Constant)
Keratin
Unstandardized
Standardized
Coefficients
Coefficients
B
Std. Error
Beta
2.055
.276
.929
.118
.627
t
7.445
7.887
95% Confidence Interval for B
Correlations
Sig.
Lower Bound Upper Bound Zero-order Partial
.000
1.507
2.603
.000
.695
1.163
.627
.627
a. Dependent Variable: Adaptation
Coefficient Correlations a
Model
1
Correlations
Covariances
Keratin
Keratin
Keratin
1.000
.014
a. Dependent Variable: Adaptation
2. SPSS(GP) calculations for multiple regression re two models of criterion
variables vs keratin.
Regression
Part
.627
137
De scriptive Stat istic s
Ke ratin
Re make
Re turn
Ag e
Ge nder
Fitting
Typ e
Me an
2.2 245
1.1 122
1.0 510
70 .295 9
1.3 673
1.5 816
1.3 163
Std . De viatio n
.73 961
.31 729
.22 117
12 .745 09
.48 456
.49 583
.46 743
N
98
98
98
98
98
98
98
Correlations
Pearson Correlation
Sig. (1-tailed)
N
Keratin
Remake
Return
Age
Gender
Fitting
Type
Keratin
Remake
Return
Age
Gender
Fitting
Type
Keratin
Remake
Return
Age
Gender
Fitting
Type
Keratin
1.000
-.372
-.386
.089
.026
-.219
.031
.
.000
.000
.191
.398
.015
.381
98
98
98
98
98
98
98
Remake
-.372
1.000
.652
.022
-.204
.039
-.033
.000
.
.000
.414
.022
.350
.372
98
98
98
98
98
98
98
Return
-.386
.652
1.000
.115
-.080
.009
-.058
.000
.000
.
.129
.215
.466
.285
98
98
98
98
98
98
98
Age
.089
.022
.115
1.000
-.044
.322
.112
.191
.414
.129
.
.332
.001
.136
98
98
98
98
98
98
98
Gender
.026
-.204
-.080
-.044
1.000
-.169
-.109
.398
.022
.215
.332
.
.048
.143
98
98
98
98
98
98
98
Fitting
-.219
.039
.009
.322
-.169
1.000
-.001
.015
.350
.466
.001
.048
.
.495
98
98
98
98
98
98
98
Type
.031
-.033
-.058
.112
-.109
-.001
1.000
.381
.372
.285
.136
.143
.495
.
98
98
98
98
98
98
98
138
Va ria b le s Ent e re d/Re mo veb
d
Mo del
1
2
Va riab les
En tere d
Re turn , a
Re mak e
Fit ting ,
Ty pe,
Ge nde
r,
a
Ag e
Va riab les
Re moved
Me tho d
.
En ter
.
En ter
a. Al l req ues ted varia bles ent ered .
b. De pen den t Va riabl e: K erat in
Mo del Su mm ary
Model
1
2
R
.417a
.515b
R Square
.174
.265
Adjust ed
R Square
.157
.217
St d. E rror of
the Es timate
.67918
.65464
a. Predic tors: (Constant), Ret urn, Rem ake
b. Predic tors: (Constant), Ret urn, Rem ake, Fit ting, Type,
Gender, Age
ANOV Ac
Model
1
2
Regres sion
Residual
Total
Regres sion
Residual
Total
Sum of
Squares
9.239
43.822
53.061
14.063
38.998
53.061
df
2
95
97
6
91
97
Mean Square
4.620
.461
F
10.015
Sig.
.000a
2.344
.429
5.469
.000b
a. Predic tors : (Const ant), Return, Remak e
b. Predic tors : (Const ant), Return, Remak e, Fitting, Ty pe, Gender, Age
c. Dependent Variable: K erat in
139
Coefficientsa
Model
1
(Constant)
Remake
Return
2
(Constant)
Remake
Return
Age
Gender
Fitting
Type
Unstandardized
Coefficients
B
Std. Error
3.644
.337
-.489
.287
-.833
.411
3.757
.582
-.449
.283
-.973
.403
.013
.006
-.120
.143
-.438
.144
-.041
.145
Standardized
Coefficients
Beta
-.210
-.249
-.193
-.291
.221
-.079
-.293
-.026
t
10.817
-1.704
-2.026
6.453
-1.585
-2.418
2.281
-.840
-3.039
-.284
95% Confidence Interval for B
Correlations
Sig.
Lower Bound Upper Bound Zero-order Partial
.000
2.975
4.312
.092
-1.058
.081
-.372
-.172
.046
-1.650
-.017
-.386
-.204
.000
2.601
4.914
.116
-1.011
.114
-.372
-.164
.018
-1.773
-.174
-.386
-.246
.025
.002
.024
.089
.233
.403
-.405
.164
.026
-.088
.003
-.724
-.152
-.219
-.304
.777
-.328
.246
.031
-.030
Part
-.159
-.189
-.142
-.217
.205
-.075
-.273
-.026
a. Dependent Variable: Keratin
Excluded Variables b
Model
1
Age
Gender
Fitting
Type
Beta In
.125a
-.038a
-.209a
.010a
t
1.332
-.398
-2.291
.103
Sig.
.186
.692
.024
.919
a. Predictors in the Model : (Constant), Return, Rem ake
b. Dependent Variable: Keratin
Partial
Correlation
.136
-.041
-.230
.011
Collinearity
Statisti cs
Tolerance
.982
.954
.998
.997
140
APPENDIX G
Video Otoscopic Views & Notes
Video otoscopic view #1
The “in-grown toenail of the
ear” left after the cerumen
has been removed…
A view of the TM after the “ingrown toenail” has been
removed in the same ear…
DigiCare®
141
Video otoscopic view #2
Advanced tympanosclerosis
with stress scar overlay
Same ear 30 days later,
Miracell used for 14 days
DigiCare®
142
Video otoscopic view #3
Healed perforation scars with
tympanosclerosis/otosclerosis
Osteoma & exostosis plus TM
scar tissue re past ear infections
DigiCare®
143
Video otoscopic view #4
Blue drum before using
Miracell botanical solution
Same ear after 14 days’ use
of Miracell (30dB rise in low Hz)
DigiCare®
144
Video otoscopic view #5
Inactive (subclinical)
cholesteatoma existing since
childhood
Insect imbedded in cerumen &
keratin before removal
DigiCare®