* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electronics
Survey
Document related concepts
Transcript
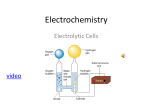
Basic Electrochemistry What is electrochemistry? • Electronics: the transport of electrons (or positive holes) Optoelectronics: light + electronics • Electrochemical systems (electrodics + ionics) • Electrochemistry: the coupling of chemical changes to the passage of electricity ionic conduction (flow of ions) + electronic conduction (flow of electrons) Electrochemical devices & electrochemical technologies Materials & devices & processings • Examples of Electrochemical devices/technologies Battery or Fuel cell: chemical state changes(electrochemistry) electric power Photoelectrochemical cell (Solar cell): light + electrochemistry electric power Photocatalysis: light hydrogen or chemical reaction Electrochromic display: chemical state changes by electric signal coloration Sensors: chemical state changes by mass electric signal Electrolysis: electric power chemical species by chemical state changes Electrodeposition: electric power chemical change: thin film, Cu metallization Several distinct states may correspond to the same energy. That is, each energy level may be degenerate. Three energy levels are shown here, possessing one, three, and five distinct states. Corrosion: potential difference chemical change Etching • Solid State Electrochemistry Solid electrolyte: solid substances which can conduct electric current by ionic motion as do electrolyte solutions “solid state electrochemistry” or “solid state ionics” “solid state device” Basic concepts for electrochemistry • Electric charge & current Electric charge (=amount of electricity) Q (unit: Coulomb, C), time t Electric current (unit: ampere (A)): I = dQ/dt Q = Idt Current density (unit: A/m2): i = I/A, A: surface of area Ammeter: measuring current Circuit: electric current flows in a closed path Electrical potential & electric field Electrical potential (unit; volts, V), : the pressure of the electric fluid Voltage: the electrical potential difference () Voltmeter: measuring an electrical potential difference Electric field strength (unit: V/m) X = -d/dx Ohm’s law: most conductors obey this law Current density is proportional to the field strength iX i = X = - d/dx ; electrical conductivity (siemens/m, S = A/V), 1/; resistivity = -RI R;resistance (unit of ohm), G; conductance, G = 1/R = A/L = -I/ L; conductor length, A; cross section Ohm’s law does not have universal validity. It does not apply to electrochemical cells. Resistor: a device that is fabricated to have a stable and known resistance Power (watts) = I2R Electrical quantities & their SI units Quantity Unit Current (I) Current density (i) Charge (Q) Charge density () Potential () Field strength (X) Conductivity () Resistance (R) Conductance (G) Permittivity () Energy of work (w) Power Capacitance (C) Ampere (A) Ampere per square meter (A/m2) Coulomb (C = As) Coulomb per cubic meter (C/m3) Volt (V = J/C) Volt per meter (V/m) Siemens per meter (S/m) Ohm ( =1/S = V/A) Siemens (S = A/V) Farad per meter (F/m = C/Vm) Joule (J = VC) Watt (W = J/s = AV) Farad (F = s/ = Ss), F = C/V Classes of conductors Materials 1.Conductors Electronic conductors Ionic conductors 2. Insulators Conductors: metals Insulators: plastics, ceramics, gases No clear cut distinction between conductor and insulator Typical value of electrical conductivity Material /Sm-1 Ionic conductors Ionic crystals Solid electrolytes Strong(liquid) electrolytes 10-16 – 10-2 10-1 – 103 10-1 – 103 Electronic conductors Metals Semiconductors Insulators 103 – 107 10-3 – 104 <10-10 S/m x10-2 for S/cm Electrical conductivity of various materials (most at 298 K) Material /Sm-1 Superconductors (low temp) Ag Cu Hg C (graphite) Doped polypyrrole Molten KCl (at 1043 K) 5.2 M H2SO4 (battery acid) Seawater Ge 0.1 M KCl H2O Typical glass Teflon, (CF2)n Vacuum & most gases 6.3 x 107 6.0 x 107 1.0 x 106 4 x 104 6 x 103 217 82 5.2 2.2 1.3 5.7 x 10-6 3 x 10-10 10-15 0 Charge carriers Electron pairs Electrons Electrons Electrons Pi electrons Pi electrons K+ and ClH+ and HSO4Cations & anions Electrons and holes K+ and ClH+ and OHUnivalent cations ? Electronically conductive polymers Mobilities: conduction from the standpoint of the charge carriers Electric current = rate at which charge crosses any plane = [number of carriers per unit volume][cross sectional area][charge on each carrier][average carrier speed] I = dQ/dt = (NAci)(A)(Qi)(i) i: particular charge carrier, ci; concentration, Qi; charge, i; average velocity, NA; Avogadro’s constant (6.0220 x 1023 mol-1), A; area zi; charge number = Qi/Qe where Qe (1.6022 x 10-19 C), e.g., electrons:-1, Mg2+; +2 i fi X d/dx fi; force exerted on the charge carrier, X; electric field strength mobility of the carrier, ui (m2s-1V-1 unit) = velocity to field ratio (i / X) i = uiX = - (zi/zi)uid/dx zi: absolute value of the charge number ue- of electrons: 6.7 x 10-3 m2s-1V-1 for Ag, less mobile in other metals mobility of ions in aqueous solution: smaller than the factor of 105 (factor 105 slower); ucu2+o = 5.9 x 10-8 m2s-1V-1 in extremely diluted solution Current I, I = -A NAQeziuicid/dx Faraday constant F = NAQe = (6.02 x 1023 mol-1)(1.6022 x 10-19 C) = 96485 Cmol-1 is numerically equal to the charge carried by one mole of univalent cations. (F is large. Small amount of chemicals higher electricity) If there are several kind of charge carriers, I = -AFd/dxziuici i = -Fd/dxziuici Transport number ti; the fraction of the total current carried by one particular charge carrier ti = (ziuici )/(ziuici) From i = X = -d/dx, conductivity = Fziuici molar ionic conductivity (i); Fui Ion mobilities at extreme dilution in aqueous solution at 298 K Ion uo/m2s-1V-1 H+ K+ Ag+ Cu2+ Na+ Li+ OHSO42ClClO4C6H5COO- 362.5 x 10-9 76.2 x 10-9 64.2 x 10-9 58.6 x 10-9 51.9 x 10-9 40.1 x 10-9 204.8 x 10-9 82.7 x 10-9 79.1 x 10-9 69.8 x 10-9 33.5 x 10-9 Capacitance parallel conducting plate separated by a narrow gap containing air or insulator Idt = Q E Q = -CE C; capacitance (unit; farads (F) = C/V) C = -Q/E = A/L A;cross-section area of the gap, L; width, ; permittivity of the insulator • Relative permittivity (r) or dielectric constant (유전상수) air: ~ 1 water: 78 Coulomb interaction energy is reduced by two orders of magnitudes from its vacuum value polar molecules: r refractive index: nr = r1/2 at the frequency Capacitor; ; current integrator Permittivity of various materials Material 1012 /Fm-1 Material 1012 /Fm-1 vacuum (0) N2(g) Teflon(s), (CF2)n CCl4(l) Polyethene (s) Mylar (s) SiO2(s) Typical glass (s) C6H5Cl(l) 8.85419 8.85905 18 19.7 20 28 38.1 44 49.8 Neoprene ClC2H4Cl(l) CH3OH(l) C6H5NO2(l) CH3CN(l) H2O(l) HCONH2(l) TiO2(s) BaTiO3(s) 58 91.7 288.9 308.3 332 695.4 933 1500 110000 /0; relative permittivity or dielectric constant mylar; poly(ethylene glycol terephthalate), (CH2OOCC6H4COOCH2)n Liquid > solid: large capacitance in electrochemical capacitor (supercapacitor) Summary Electricity flows either by electron motion or ion motion In both cases, the intensity of the flow (= current density) electric field strength conductivity i = X = -d/dx = Fziuici determined by the concentration of charge carriers and their mobilities one form of Ohm’s law E = -RI potential difference across resistor to the current flowing through it Resistor: dissipate energy Capacitor: store energy . Potential & Thermodynamics Introduction Electrochemistry: chemical change electric force Electrodics: in which the reactions at electrodes are considered Ionics: in which the properties of electrolytes have the central attention concentration of ions, their mobilities, interactions etc Basic laws were developed in systems with liquid electrolytes “solid state” (same and different features of solid electrolyte system) Ionic solutions Most important ionic conductor e.g., aqueous solution of electrolyte Electrolyte; a substance that produces ions so enhance the electrical conductivity e.g., solid(NaCl), liquid(H2SO4), gas(NH3) cf) solid electrolyte Electrode The junction between electronic conductor and ionic conductor that the chemistry of electrochemistry occurs Electrochemical cell Basic unit: an ionic conductor sandwiched between two electronic conductors e.g., aqueous solution of electrolyte between two pieces of metal, solid electrolyte between two metals Cell voltage (E) or emf(electromotive force) electric potential difference between the two electronic conductors voltameter e.g., lead/acid cell (car battery) Electronic conductors: PbO2, Pb Ionic conductor: concentrated aqueous solution of sulfuric acid Electrochemical reaction Anode: Pb(s) + HSO4-(aq) 2e- + PbSO4(s) + H+(aq) Cathode: PbO2(s) + HSO4-(aq) + 3H+(aq) + 2e- PbSO4(s) + 2H2O(l) Cell: PbO2(s) + Pb(s) + 2H+(aq) + 2HSO4-(aq) 2PbSO4(s) + 2H2O(l) Right-hand electrode: electrons produced: oxidation, “anode” Left-hand electrode: electrons consumed; reduction, “cathode” Energy is delivered by the cell into the load; ex) car: starting engine, lighting lamps Galvanic cell: a cell which provides energy in this way, “discharge”(방전) 2.0 V without current flow, 1.8 V with current flow (load); “polarization”; voltages decrease in magnitude when energy is taken from them. the effect becomes greater if the current is increased. “charge” (충전): current flow in the opposite direction by using an external source (ex. Battery); Electrolytic cell; opposite direction to its spontaneous motion PbO2 : anode, Pb: cathode 2.0 V; perfect balance between the applied and cell voltages, no current flow equilibrium cell voltage or reversible cell voltage or null voltage or rest voltage or “open-circuit voltage”(since no current flows, it makes no difference if the circuit is interrupted, as by opening the switch) Voltammogram Plot of cell currents versus the cell voltages (volt + am(pere) + mogram) Not linear electrochemical cells do not obey Ohm’s law Notation of the structure of cells Zn/Zn2+, Cl-/AgCl/Ag Hg/Hg2Cl2/Cl-(aq)//Zn2+(aq)/Zn /: phase boundary, “,” or : two components in the same phase, //: liquid junction (a salt bridge) left: oxidation (anode), right: reduction(cathode) Thermodynamics Why is it that chemical reactions in electrochemical cells proceed spontaneously in one direction and furnish current? (thermodynamics: 평형상태에 대한 정보, kinetics: 전극반응속도에 대한 정보) : Cell potential of an electrochemical cell Ecell = Eright – Eleft or Ecell = Ecathode – Eanode E obtained from the Nernst equation oO + …+ ne- = rR + …. pP + …. = qQ + … + neoO + pP + … = qQ + rR + … (reduction) (oxidation) Ecell (cell reaction) Ecell = E0 – (RT/nF)ln[(aQqaRr..)/(aOoaPp..)] Gibbs free energy, G = -nFEcell G <0 spontaneous E0: standard electrode potential = Eright0 – Eleft0 Eright0, Eleft0,,: standard electrode potential of half reactions expresses as reductions vs. NHE(normal hydrogen electrode) with all species at unit activity (ai =1) (see the Table of Standard Potentials) e.g., MnO2 + 4H+ + 2e- Mn2+ + 2H2O E0 = + 1.23 V E = E0 –(RT/2F)ln[(aH+4)/aMn2+], G = -nFE aMnO2, aH2O = unity cf. RT/2F = [(8.314 JK-1mol-1)(298 K)/2(96485 JV-1mol-1)] = 0.01285 V Several distinct states may correspond to the same energy. That is, each energy level may be degenerate. Three energy levels are shown here, possessing one, three, and five distinct states. Several distinct states may correspond to the same energy. That is, each energy level may be degenerate. Three energy levels are shown here, possessing one, three, and five distinct states. e.g., Zn/Zn2+(aq), Cu2+(aq)/Cu Cell: Zn + Cu2+ Zn2+ + Cu Right: Cu2+ + 2e- Cu E0 = +0.34 V Left: Zn2+ + 2e- Zn E0 = -0.76 V Ecell0 = +0.34 – (-0.76) = +1.10 V G0 = -2 x 1.10(V) x 96485 (JV-1mol-1) = -212 kJmol-1 reaction spontaneous EEcell = E0 – (RT/2F)ln(aZn2+/(aCu2+) If we assume aZn2+= aCu2+, Ecell = 1.10 V -----Hg/Hg2Cl2/Cl-(aq)//Zn2+(aq)/Zn 2Hg + Cl- + Zn2+ Hg2Cl2 + Zn right: Zn2+ + 2e- Zn E0 = -0.76 V left: Hg2Cl2 + 2e- 2Hg + 2Cl- E0 = +0.27 V Ecell0 = -0.76 –0.27 =-1.03 V, G0 = +199 kJmol-1, should be opposite direction Measurement of E0: (i) experiment (ii) E0 = (RT/nF)lnK, K; equilibrium constant of cell K = exp(-G0/RT) (iii) E0 = Eright0 – Eleft0 or E0 = Ecathode0 – Eanode0 (from Table) (iv) E0 = -G0/nF Cell: PbO2(s) + Pb(s) + 2H+(aq) + 2HSO4-(aq) 2PbSO4(s) + 2H2O(l) From thermodynamics Table, Standard Gibbs Energy (kJmol-1): -813.76 (PbSO4(s)), -237.13 (H2O(l)), -218.96 (PbO2(s)), -755.91 (HSO4-(aq)), cf) G0 for element (Pb(s)) and H+(aq) = 0 G0 = 2G0 (PbSO4(s)) + 2G0 (H2O(l)) – [G0 (PbO2(s)) + 2G0 (HSO4-(aq))] = -371 kJmol-1 G0 = -nFE0 E0 = 371000(Jmol-1)/[2 x 96485 (JV-1mol-1)] = 1.923 V battery acid: 5.2 M Ecell = 1.923 V – (RT/2F)ln[aH2O(l)2/(aH+(aq)2aHSO4-(aq)2)] = 1.923 V – 0.01285ln [1/(5.2)2] = 2.008 V activity term: minor contribution to the cell voltage activity (a) concentration (c); a = c, ; activity coefficient ai 1(solvent, pure solid, ideal solution) (Examples) 1. Indicate in the following reactions which are reductions and which are oxidations: (1) Fe2+ + 2e- Fe (2) Cl- 1/2Cl2 + e- (3) Fe2+ Fe3+ + e(4) CrO42- + 3e- Cr3+ (5) O2 + 4e- 2O2(6) Br2 + 2e- 2Br2. A Galvanic cell is constructed from a Cu2+/Cu electrode and an Ag+/Ag electrode. (1) Make a schematic drawing of the cell (2) Write the reactions at the electrode (3) Indicate the anode and the cathode 3. Assuming standard states for all reactants and products, determine the spontaneous direction of the following reactions by calculating the cell potential: (1) Cu + 2HCl = CuCl2 + H2 (2) Ag + FeCl3 = FeCl2 + AgCl Principles of electrochemistry: Definitions Two equal electrodes (Lecture #4) interest in one electrode only (Lecture #5) Electrodes Working electrode(WE): electrode of interest Reference electrode(RE): second electrode, measure potential of WE with respect to RE Electrode potential E = Ework –Eref Reference electrodes SHE (standard hydrogen electrode) or NHE(normal hydrogen electrode): universally accepted standard H+(aq, a=1) + e- = 1/2H2(g, 105 Pa) E = 0 V SCE (saturated calomel electrode) Hg2Cl2(s) + 2e- = 2Hg + Cl- Eref = 0.244 V vs. NHE Ag/AgCl AgCl(s) + e- = Ag(s) + Cl-(aq) Eref = 0.199 V with saturated KCl Potentials of reference electrodes E(RHE) = E(NHE) + 0.05916pH E(SCE) = E(NHE) – 0.2444 E(Ag/AgCl) = E(NHE) – 0.2223 E(Ag/AgCl, sat.KCl) = E(NHE) – 0.196 E(Hg/HgO 1M KOH) = E(NHE) – 0.1100 + 0.05946pH E(Hg/Hg2SO4) = E(NHE) – 0.6152 Potential vs. energy (vs. vacuum) 예: Potential vs. energy (vs. vacuum) Controlling potential of the working electrode with respect to the reference controlling the energy of the electrons within the working electrode More negitive potential energy of electrons is raised reach a level to occupy vacant states (LUMO) on species in the electrolyte flow of electrons from electrode to solution (a reduction current) More positive potential electron flow from solution (HOMO) to electrode (oxidation current) Working electrode can act (i) as only a source (for reduction) or a sink (for oxidation) of electrons transferred to or from species in electrolyte (e.g., C, Au, Pt, Hg) or can (ii) take part in the electrode reaction, as in dissolution of a metal M (Zn Zn2+ + 2e-) Applying potential from its equilibrium (or its zero-current) Polarization Voltammogram: historical one vs. new one E > 0 working electrode potential > 0 (positive: right of x-axis) I > 0 oxidation at the working electrode Polarization: the shift in the voltage across a cell caused by the passage of current Departure of the cell potential from the reversible(or equilibrium or nernstian) potential Ohmic polarization Activation polarization Concentration polarization Overvoltage (): the voltage shift caused by each kind of polarization Extent of potential measured by the overpotential: = E - Eeq E = En + ohm + act + conc (i) ohmic polarization ohm = IRsol, “IR drop” If free of activation & concentration polarization, slope = 1/Rsol Rsol = L/A Rsol = L/A If free of activation & concentration polarization, slope = 1/Rsol Electrochemistry needs to minimize ohm (conductivity) ohm (by adding extra electrolyte: “supporting electrolyte”) three-electrode system two-electrode cell vs. three-electrode cell Eappl = E + iRs = Eeq + + iRs IRs: ohmic drop in the solution (ohmic polarization) should be minimized short distance between working and reference electrode & three-electrode cell Two-electrode cell: iRs problem due to high current flow Three-electrode cell: current between WE and auxiliary electrode(or counter electrode) Potential measurement between WE and RE almost no current to reference electrode Potentiostat, etc electrochemical system: three electrode system (ii) activation polarization slow electrode reaction activation polarization; slow kinetics activation energy This can be overcome by increasing the temperature and by applying extra voltage (activation overvoltage (act)) (iii) concentration polarization from difference between the electrode surface and bulk concentration R O + neconc = E –En = (RT/nF)ln[(cRbcOs)/cRscOb]] Limiting current Ideal polarizable electrode (totally polarized electrode): a very large change in potential upon small current Ideal nonpolarizable electrode: potential does not change upon passage of current (e.g., reference electrode) Double layer Electrode-solution interface capacitor “double layer” Same concept as capacitor (two metal sheets separated with q (coulomb)/E = C(farad)) )) Same concept as capacitor (two metal sheets separated with q (coulomb)/E = C(farad)) )) qM = -qS qM: charge from electrons in metal electrode, qS: charge from ions in solution charge density M =qM/A (C/cm2) double layer capacitance, cd: 10 ~ 40 F/cm2 several models: Helmholtz, Gouy-Chapman, Stern, Grahame model etc Grahame model: IHP (inner Helmholtz plane, specifically adsorbed anion) + OHP (outer HP, solvated cation) + diffuse layer Electrochemical systems in terms of circuit elements e.g.,) Hg/K+, Cl-/SCE, Hg: ideal polarized electrode CSCE, Cd: capacitances of SCE and double layer, Rs: solution resistor CT = CSCECd/(CSCE + Cd), CSCE Cd CT Cd RC circuit e.g.,) applying voltage (or potential) step: potential step: E, EC of capacitor, ER of resistor q = CdEC E = ER + EC = iRs + q/Cd i = dq/dt dq/dt = -q/(RsCd) + E/Rs q =0 at t = 0 q = ECd[1 – exp(-t/RsCd)] By differentiating, I = (E/Rs)exp(-t/RsCd) At time constant = RsCd current for charging the double layer capacitance drops to 37 % at = t, 5 % at = 3t e.g.,) Rs = 1 , Cd = 20 F, = 20 sec double layer charging is 95 % complete in 60 sec sec sec Double layer charging pr Double layer charging process: “non-faradaic process” Cf) oxidation /reduction electron transfer ; governed by Faraday’s law (the amount of chemical reaction caused by the flow of current is proportional to the amount of electricity passed) “faradaic process” or “charge transfer process” Semiconductor electrode Semiconductor/electrolyte space charge region due to space charge capacity, Csc, 0.001 ~ 1 Fcm-2, (cf; Cdl = 10 ~ 100 Fcm-2 ) band bending n-type SC when EF of SC lies above that in electrolyte electron flow from SC (positively charged) to electrolyte (negatively charged) bent upward by applying potential of bulk = surface, band bending & space charge region disappear “flat band potential (fb or Efb)” space charge capacitance Csc Mott-Schottly equation 1/Csc2 = (2/e0N)1/2(- - kT/e) : dielectric constant, N: donor or acceptor densities, e: quantity of charge, - = E-Efb A plot of 1/Csc2 vs. potential E should be linear Efb, doping level N p-type p-type