* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2 - My George School
Electric charge wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Periodic table wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Livermorium wikipedia , lookup
Elementary particle wikipedia , lookup
Stoichiometry wikipedia , lookup
Metallic bonding wikipedia , lookup
Electronegativity wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical element wikipedia , lookup
Extended periodic table wikipedia , lookup
Atomic orbital wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Hydrogen atom wikipedia , lookup
History of chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Metalloprotein wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical bond wikipedia , lookup
Electron configuration wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Atomic nucleus wikipedia , lookup
Isotopic labeling wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
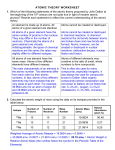
Dalton’s Atomic Theory and Atomic Basics Ch 4: 4.1 to 4.7 Element, Compound, or Molecule? Element- grab your ear like so Compound- clap as shown Molecule-move to the groove H2: ________ MgO: _______ Fe: _________ Na2SO4 : _______ Hg: _________ KI: __________ 1. Elements are made of tiny particles called ________ 2. All atoms ____________________ 3. The atoms of a given element are _______________________________ 4. Atoms of one element can combine with _______________ to form __________. A given compound always has the same _______________________________ 5. Atoms are not __________ or __________ in chemical reactions. Bonds can _______ and _______, but _______ cannot 2 Mg(s ) + O2(g ) --> 2 MgO(s ) Your turn Everyone Responds • sulfur has 16 total electrons, while nitrogen has only 7 • Potassium iodide (KI) combines with lead nitrate (Pb(NO3)2) to form solid lead iodide (PbI2) reaction • Hydrogen gas, H2, contains only H atoms • The balanced chemical reaction of magnesium with oxygen is below: 2 Mg(s ) + O2(g ) --> 2 MgO(s ) Formulas of Compounds In a chemical formula, atoms are indicated by element __________ and the numer of each type of atom is indicated by a numerical _________. The state of matter is often indicated by _______________. Examples: CaCl2(s) C6H6(l) The modern view of the atom Opposite charges attract, like charges repel. The nucleus of an atom contains: ____________________ ____________________ Electron cloud contains ____________________________ The net charge of any atom or compound is ______ Masses of Particles Proton = ________ amu Neutron = _______ amu Electron = _______ amu This means that the majority of an atom’s mass comes from _______ and _______. Rutherford’s Experiment- 1911 • Alpha particles shot at a thin gold foil • Some are _______, some go ________, some __________! – Proves atoms have positively charged nucleus – Disproves Lord Kelvin’s __________________ Vocab time! Atomic number: # of _________ in the nucleus Mass number: sum of # of ________ + # of ___________ Find the atomic number of each of the following: B, Au, Be, C Practice problems Isotopes Atoms with the same number of ________ but different numbers of ___________ Questions: What is the charge on an isotope? Will the mass number of the isotope be different than that of the more naturally abundant atom? Why aren’t the masses listed on the periodic tables whole numbers? Calculating average atomic mass: Weighted average: (% abund. in dec. form)*(atomic mass/weight) +…. Example #4: Silicon mass number exact weight percent abundance 28 27.976927 92.23 29 28.976495 4.67 30 29.973770 3.10 The answer for silicon: 28.086 Try this one on your own! Isotope name Isotope mass (amu) Silver-107 106.90509 percentage 51.86 Silver-109 108.90470 remainder The Cathode Ray Tube J.J. Thompson discovered that atoms contain electrons, using a CRT diagram: