* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The evolving landscape of MS
Survey
Document related concepts
Transcript
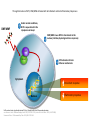
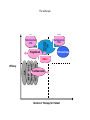
The evolving landscape of MS John Woolmore Consultant Neurologist UHB FIN14-C173. Date of preparation: September 2014 Plan • Introduction • Established therapies • Newer therapies, “on the horizon” • MOA, pivotal trials, other efficacy data, safety • NHS England • Conclusion and questions Putative targets for MS therapies Barten et al Drug Des Devel Ther. 2010;4:343-366; Linker et al, Trends Pharmacol Sci. Established therapies • ABCR/BRACE • Tysabri • Gilenya • (mitoxantrone) Newer therapies • Lemtrada, Alemtuzumab, Campath • Tecfidera, Dimethyl fumarate, BG 12 • Aubagio, Teriflunomide, (leflunomide) On the horizon • Daclizumab - anti IL-2 • Ocrelizumab • Firategast alpha4beta1 integrin • Ofatumumab • BAF 312 - S1P1 and S1 P5 • Anti Lingo BIIB033 • Copaxone/glatriamer TIW • Plegridy 2 weekly IFN beta1a sub cut • (Laquinimod) Daclizumab • DECIDE - Presented 11/9/14 ECTRIMS 2014, Kappos et al • DAC HYP once monthly s/c vs IFNB1a • 45% reduction in ARR • Relapse risk reduction rate 41% • T2 MRI rate 54% reduction, GAD 65% • 6 month disability progression reduced by 27% • Treatment discontinuation 9% - cutaneous events main AEs Tecfidera Sustained-Release Dimethyl Fumarate Through Activation of Nrf2, DMF/MMF Activate Both Anti-Oxidant and Anti-Inflammatory Responses DMF/MMF Under normal conditions, 1 Nrf2 is sequestered in the cytoplasm via Keap1 2 DMF/MMF cause Nrf2 to translocate to the nucleus (imitates physiological stress response) Nrf2 Keap1 Nrf2 activates intrinsic 3 defense mechanisms Cytoplasm Antioxidant response Nucleus Nrf2=nuclear factor (erythroid-derived 2)-like 2; Keap1=kelch-like ECH-associated protein. van Horssen J et al. Biochem Biophys Acta. 2011;1812:141-150; Linker RA et al. Brain. 2011;134:679-692; Scannevin R et al. J Pharmacol Exp Ther. 2012;341:274-284. Inflammatory response DEFINE: Proportion of Patients Relapsed (INEC-Confirmed) 0.6 Probability of Relapse 0.5 HR (95% CI): BID vs placebo=0.51 (0.40, 0.66); 49% risk reduction; P<0.0001 TID vs placebo=0.50 (0.39, 0.65); 50% risk reduction; P<0.0001 0.461 0.4 0.3 0.2 0.270 0.260 0.1 0 Placebo (n=408) DMF 240 mg BID (n=410) DMF 240 mg TID (n=416) BL 12 24 36 48 60 Time on Study (weeks) Number of 243 Patients at Risk 282 224 Placebo408 356 321 DMF BID410 353 324 303 286 DMF TID416 346 322 301 286 72 84 96 205 190 115 267 255 243 154 270 251 244 166 DEFINE=Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS; INEC=independent neurology evaluation committee; HR=hazard ration; CI=confidence interval; BID=twice daily; TID=3 times daily; BL=baseline; MS=multiple sclerosis. Gold R et al. Presented at ECTRIMS; October 19–22, 2011; Amsterdam, The Netherlands. S34. DEFINE: Annualized Relapse Rate at 2 Years (Secondary Endpoint) 53% reduction vs placebo P<0.0001 Adjusted Annualized Relapse Rate (95% CI)* 0.6 48% reduction vs placebo P<0.0001 0.364 0.172 0.189 0. Placebo (n=408) BG-12 BID (n=410) BG-12 TID (n=416) *Annualized relapse rate calculated with negative binomial regression, adjusted for baseline EDSS score (≤2.0 vs >2.0), baseline age (<40 vs ≥40 years), region, and number of relapses in the 1 year prior to study entry. DEFINE=Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS; CI=confidence interval; BID=twice daily; TID=3 times daily; EDSS=Expanded Disability Status Scale; MS=multiple sclerosis. Gold R et al. Presented at ECTRIMS; October 19–22, 2011; Amsterdam, The Netherlands. S34. Integrated Safety Analysis: Common Adverse Events (≥10% in Any Group) Event, n (%) Placebo (n=836) DMF bid (n=769) DMF tid (n=823) Any adverse event 769 (92) 733 (95) 767 (93) 39 (5) 265 (34) 240 (29) MS relapse 360 (43) 221 (29) 211 (26) Nasopharyngitis 169 (20) 170 (22) 179 (22) Headache 137 (16) 133 (17) 138 (17) Diarrhea 86 (10) 107 (14) 136 (17) Urinary tract infection 96 (11) 107 (14) 95 (12) Upper respiratory tract infection 88 (11) 99 (13) 101 (12) Nausea 72 (9) 93 (12) 115 (14) Fatigue 91 (11) 94 (12) 103 (13) Back pain 92 (11) 94 (12) 84 (10) Abdominal pain upper 47 (6) 76 (10) 94 (11) Proteinuria* 59 (7) 67 (9) 85 (10) Flushing Indicates ≥3% higher incidence in either dimethyl fumarate group vs placebo. DMF 240mg BID is the licensed dose , 240mg BID and 240mg TID DMF doses were assessed for safety and efficacy in DEFINE and CONFIRM. The overall incidence of any GI event was 31%, 40%, and 43% in the placebo, dimethyl fumarate bid, and dimethyl fumarate tid groups, respectively; *no notable differences in levels of BUN and creatinine, urine β2-microglobulin, and urine microalbumin were observed across treatment groups with monitoring every 4 weeks.GI=gastrointestinal; BUN=blood urea nitrogen. Selmaj K et al. Presented at ECTRIMS; October 10–13, 2012; Lyon, France. P484. Integrated Safety Analysis: Events Leading to Study Drug Discontinuation (≥1% in Any Group) Placebo (n=836) DMF bid (n=769) DMF tid (n=823) Discontinued study drug due to adverse event 94 (11) 109 (14) 117 (14) MS relapse 48 (6) 11 (1) 13 (2) Flushing 1 (<1) 24 (3) 13 (2) Event, n (%) GI events 4% discontinuation for GI events in DMF BID group Diarrhea 2 (<1) 7 (<1) 15 (2) Nausea 0 6 (<1) 14 (2) Vomiting 0 8 (1) 12 (1) 2 (<1) 6 (<1) 10 (1) 0 5 (<1) 9 (1) Abdominal pain, upper Abdominal pain GI=gastrointestinal. DMF 240mg BID is the licensed dose , 240mg BID and 240mg TID DMF doses were assessed for safety and efficacy in DEFINE and CONFIRM. Selmaj K et al. Presented at ECTRIMS; October 10–13, 2012; Lyon, France. P484. Natalizumab AFFIRM: Annualized Relapse Rate Overall Population 68% reduction in annualized relapse rate vs placebo 1.0 Annualized Relapse Rate (95% CI) 0.9 0.8 0.7 Placebo n=315 Natalizumab n=627 0.78 0.73 68% reduction vs placebo P<0.001 66% reduction vs placebo P<0.001 0.67 70% reduction vs placebo P<0.001 0.6 0.5 0.4 0.3 0.27 0.23 0.20 0.2 0.1 0 Years 0–2 CI=confidence interval. Polman CH et al. N Engl J Med. 2006;354:899-910. 5 Year 0–1 Years 1–2 AFFIRM: Risk of Disability Progression Overall Population 6-Month Sustained† 0.4 42% risk reduction HR=0.58 P<0.001 Placebo 29% 0.3 0.2 0.1 Natalizumab 17% 0 0 12 24 36 48 60 72 Week 84 96 108 120 Proportion with Sustained Progression Proportion with Sustained Progression 3-Month Sustained* 0.4 54% risk reduction HR=0.46 P<0.001 0.3 Placebo 23% 0.2 0.1 Natalizumab 11% 0 0 12 24 36 315 627 296 601 283 582 264 567 248 546 240 525 229 517 *Primary endpoint; †prespecified sensitivity analysis. HR=hazard ratio. Polman CH et al. N Engl J Med. 2006;354:899-910. 6 Updated Sept 2012 60 72 Week 84 96 108 120 225 521 216 509 Number of Patients at Risk Number of Patients at Risk Placebo Natalizumab 48 216 503 208 490 200 478 199 473 Placebo 315 Natalizumab 627 298 611 287 594 269 581 253 559 246 540 237 532 211 503 211 502 Natalizumab: long-term efficacy data from STRATA1 Annualized Relapse Rate STRATA is an ongoing, open-label, multinational study to evaluate the long-term safety and efficacy of natalizumab in RRMS patients who completed the feeder studies AFFIRM, SENTINEL and GLANCE. 1. Rudick, et al. ECTRIMS 2013; P593. Incidence per 1000 patients Natalizumab PML Incidence Estimates by Treatment Epoch 4.0 3.99 3.64 3.5 3.32 2.97 3.0 2.68 2.65 2.5 2.05 2.0 1.72 1.42 1.5 2.21 1.82 2.41 1.93 0.85 0.67 0.51 1.0 0.5 0.13 0.07 0.03 0.0 Calculations based on exposure through 31st May 2014 and 472 confirmed cases as of 4th June 2014 Biogen Idec, data on file. 2.02 1.49 Risk Stratification Tool: The Presence of Anti-JCV Antibodies, Prior Immunosuppressant Use, Treatment Duration* Anti-JCV Antibody Status Negative Positive Prior IS Use No Natalizumab Exposure 1–24 months 0.1/1000 25–48 months 95% CI 0.01-0.35 49–72 months No Prior IS Use Yes Prior IS Use 0.7/1000 1.8/1000 95% CI 0.5-1.0 95% CI 1.1-2.7 5.3/1000 11.2/1000 95% CI 4.4-6.2 95% CI 8.6-14.3 6.1/1000 95% CI 4.8-7.8 Insufficient data Data beyond 4 years of treatment are limited. There are insufficient data to adequately determine PML risk in anti-JCV antibody positive patients with prior IS use and >48 months of natalizumab exposure. *Based on natalizumab exposure and 343 confirmed PML cases as of 5th March 2013. Prior IS data in overall natalizumab-treated patients based on proportion of patients with IS use prior to natalizumab therapy in TYGRIS as of May 2011; and prior IS data in PML patients as of 5th March 2013. The analysis assumes that 55% of natalizumab-treated MS patients were anti-JCV antibody positive and that all PML patients test positive for anti-JCV antibodies prior to the onset and diagnosis of PML. The estimate of PML incidence in anti-JCV antibody negative patients is based on the assumption that all patients received at least 1 dose of natalizumab . Assuming that all patients received at least 18 doses of natalizumab, the estimate of PML incidence in anti-JCV antibody negative patients was generally consistent (0.16/1000; 95% CI 0.02–0.56). Biogen Idec, data on file. Teriflunomide Alemtuzumab Relapse Reduction: in RRMS and SPMS Patients Relapse frequency significantly reduced in RRMS cohort (p<0.0001) 2.21/patient/yr before treatment vs. 0.19/patient/yr after treatment 91% reduction Similar results were seen in the SPMS cohort The relapse rate fell from 0.7/patient/yr to 0.01/patient/yr (p<0.001; data not shown), which translates to a 98.5% reduction 2 Relapses per 3-month Period, n Graph shows episodes reported in RRMS cohort (n=22) before and after treatment with 2 coursesa of alemtuzumab at Months 0 and 12 (red lines) Relapse Rates in RRMS Cohort 1 0 -42 -36 -30 -24 -18-12-6 0 6 12 18 24 30 Months Before Alemtuzumab Treatment Course=3 or 5 daily 20-mg intravenous administrations. Coles AJ et al. J Neurol 2006;253:98-108. a 36 42 48 After Alemtuzumab Treatment Change in EDSS: in RRMS and SPMS Patients 1 year after alemtuzumab treatment, 33/36 SPMS patients had maintained their pre-treatment EDSS values However, this proportion decreased with time and this cohort showed an overall worsening of disability Note: Gradients above the equator represent increasing disability and below the equator represent reducing disability. Coles AJ et al. J Neurol 2006;253:98-108. ‹#› Fingolimod in MS: efficacy from Phase III studies 0.20 0.10 TRANSFORMS4 1 year, RRMS (n = 1292) vs IFNβ-1a IM -82%*** (Gd+) -74%*** (T2) -30%* (3-mo conf.) -37%* (6-mo conf.) Placebo Fingolimod 1 2 (n = 418) 0.5mg (n = 425) ARR ARR 0.30 0.20 0. 40 0.10 -48%*** 0. 21 0.00 -70%*** (Gd+) -74%*** (T2) -17%, NS (3-mo conf.) -28%, NS (6-mo conf.) Placebo 1 Fingolimod 2 0.5mg (n = 355) (n = 358) 0.40 East 0.30 0. 33 ARR ARR 2 years, RRMS (n = 1083) vs placebo 0. 18 0.00 0.40 FREEDOMS II2,3 0. 40 -54%*** 0.20 0.10 0.00 -52%*** 0. 16 IFNβ-1a 1 IM Fingolimod 2 (n = 431) 0.5mg (n = 429) -55%*** (Gd+) -35%** (T2) -25%, NS (3-mo conf.) – BRAIN VOLUME LOSS Mean change in brain volume (%) ARR 2 years, RRMS (n =1272) vs placebo 0.30 ARR FREEDOMS1 DISABILITY PROGRESSION Mean change in brain volume (%) 0.40 LESIONS† Mean change in brain volume (%) RELAPSES ‹#› 0 Time (months) 6 12 24 0.0 ** –0.2 –0.4 * –0.6 n = 357 –0.8 *** –1.0 n = 331 –1.2 -35%*** –1.4 0 Time (months) 6 12 24 0.0 –0.2 –0.4 * *** n = 266 –0.6 *** –0.8 –1.0 –1.2 –1.4 0 0.0 –0.2 -33%*** n = 249 Time (months) 12 24 *** n = 368 –0.4 –0.6 n = 359 –0.8 –1.0 –1.2 -32%*** –1.4 Data are presented from separate clinical trials Caution is required when indirectly comparing studies due to differences in trial designs and populations Changes in disability progression shown here were measured using the EDSS scale. % change is vs comparator arm. *p<0.05; **p<0.01; ***p<0.001 vs placebo. †Number of Gd+ lesions or number of new / newly enlarged T2 lesions. ARR, annualised relapse rate; conf., confirmed; IM, intramuscularly; NS, not significant; RRMS, relapsing–remitting MS. 1. From N Engl J Med; Kappos L et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis, 362: 387–401. Copyright © (2010) Massachusetts Medical Society. Reproduced with permission from Massachusetts Medical Society; 2. Reproduced from Lancet Neurol 13(6) Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. pp545-56. ©2014 with permission from Elsevier; 3. Calabresi PA et al. Poster 015 presented at AAN 2012; 4. From N Engl J Med; Cohen JA et al. Oral Fingolimod or © Novartis Pharma AG 5/2014 192144 Intramuscular Interferon for Relapsing Multiple Sclerosis, 362: 402–415. Copyright © (2010) Massachusetts Medical Society. Reproduced with permission from Massachusetts Medical Society Safety Profile Safety signals of note (as per Gilenya Risk Management Plan) Treatment initiation results in transient decreases in heart rate and may be associated with atrioventricular conduction delays In clinical trials, macular oedema occurred in 0.4% of fingolimod patients Lower respiratory tract infections were more common compared to placebo Liver enzyme elevations observed, primarily during first 12 months of treatment Vaccination may be less effective during, and for up to 2 months after, treatment Hypertension was reported for 6.1% of fingolimod patients vs 3.8% placebo patients There are very limited data on the use of fingolimod in pregnant women; pregnancy should be avoided while on treatment ‹#› Incidence of adverse events in FREEDOMS Fingolimod 0.5mg (n=425) Placebo (n=418) 401 (94%) 387 (93%) 32 (8%) 32 (8%) 43 (10%) 56 (13%) 0 (0%) 2 (<1%) Headache 107 (25%) 96 (23%) Elevated liver enzyme levels 67 (16%) 21 (5%) Influenza virus infection 55 (13%) 41 (10%) Diarrhoea 50 (12%) 31 (7%) Back pain 50 (12%) 29 (7%) Fatigue 48 (11%) 45 (11%) Cough 43 (10%) 34 (8%) All adverse events Patients with ≥1 event Study drug discontinuation because of adverse event Serious adverse events Patients with ≥1 event Deaths Most common adverse events (increased incidence in fingolimod group vs placebo) This is not a complete list; please refer to prescribing information for full information. Gilenya® 0.5mg/day is the only dose approved for the treatment of MS. Kappos L et al. N Engl J Med 2010; Novartis. Gilenya® Summary of Product Characteristics. NICE/ NHSEng - Positioning • First line ABCR/BRACE/Tecfidera/Aubagio/Lemtrada • Second line - highly active - Gilenya/ Lemtrada • n.b. Gilenya post ABCR/BRACE • RES MS - Tysabri/ Lemtrada Natalizumab JCVFingolimod Alemt uzum ab The landscape Natalizumab JCV+ Mitoxantrone BG12 Efficacy Teriflunomide Burden of Therapy for Patient Questions