* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Health information technology - Welcome to the Course Materials Site!
Survey
Document related concepts
Transcript
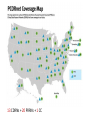
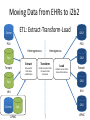
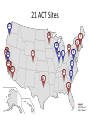
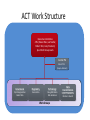
Interoperability, Health Information Exchanges and Clinical Data Research Networks Shyam Visweswaran, MD, PhD Associate Professor Biomedical Informatics Clinical and Translational Science Institute 12 May 2016 “Medicine used to be simple, ineffective and relatively safe. Now it is complex, effective and potentially dangerous.” Sir Cyril Chantler Former Dean Guy’s, King and St. Thomas’s Medical and Dental School Lancet 1999 “Current practice depends upon the clinical decision making capacity and reliability of autonomous individual practitioners for classes of problems that routinely exceed the bounds of unaided human cognition.” Daniel R. Masys, MD October 15, 2001 IOM Annual Meeting Health information technology is a critical enabler to broad transformation in health care with the ultimate goal of obtaining high quality health care. Premise of the Health Information Technology Economic and Clinical Health (HITECH) Act Health Information Technology (HIT) uses: • In the clinical enterprise to – Integrate services and reduce fragmentation of care – Improve patient safety and reduce errors – Enhance communication between providers and patients • In the research enterprise to – Leverage EHR for clinical and translational research – Enhance quality improvement and outcomes research Health Information Technology for Networks • Health Information Exchange (HIE) – Verb: HIE is the mobilization of health care information electronically across organizations within a hospital system, region or nation – Noun: HIE refers to the organization or entity that facilitates the exchange • Clinical Data Research Network (CDRN) – CDRN is a network to share standardized health information that is collected during the routine course of patient care for research • Interoperability – Is the ability to share electronic data generated by one system to be accessed and (re-)used in a meaningful way by another system (whether or not the latter system is based on different technologies) Interoperability • Interoperability describes the extent to which systems and devices can exchange data, and interpret that shared data • Interoperability in computerized healthcare information systems lags far behind other domains such as finance • Without interoperability, data and information such as patient records can't easily be shared across and sometimes within enterprises • Interoperability is critical for building networks like HIEs and CDRNs Interoperability Challenges • Health information technologies, like EHRs, where they have already been deployed, may not have been designed to support interoperability • A lot of computerized clinical data are stored in legacy systems in proprietary formats which are difficult for other systems to access, re-represent and transfer for (re)use • Many healthcare information standards to support interoperability are only just now being developed • Where healthcare information standards do exist they may also compete, making interoperability more difficult to achieve • Implementation of interoperable health information systems may require a high degree of technical expertise not readily available to small organizations in particular Three Levels of Health Information Technology Interoperability 1. Foundational interoperability – allows data exchange from one information technology system to be received by another – does not require the ability for the receiving information technology system to interpret the data 2. Structural interoperability – is an intermediate level that defines the structure or format of data exchange (e.g., the message format standards) – structural interoperability defines the syntax of the data exchange 3. Semantic interoperability – provides interoperability at the highest level, which is the ability of two or more systems or elements to exchange information and to use the information that has been exchanged – this level of interoperability defines the semantics in addition to the syntax of the data exchange – this level of interoperability is required to improve quality, safety, efficiency, and efficacy of healthcare delivery ONC • The Office of the National Coordinator for Health Information Technology (ONC): – is a division of the U.S. Department of Health and Human Services – was established in 2009, with the enactment of the HITECH Act – Is charged with formulating the federal government’s health information technology (health IT) strategy and coordinating federal health IT policies, standards, programs, and investments ONC Activities • Policy Development and Coordination – Health IT Policy – Privacy and Security – Health IT Safety and Usability • Standards, Interoperability, and Certification – – – – Standards Development and Harmonization Health Information Exchange Certification and Accreditation of EHR systems Federal Health Architecture • Adoption and Meaningful Use of Health IT – Provider Adoption Support – Consumer eHealth – Planning, Evaluation, and Monitoring Health Information Exchanges (HIEs) Benefits of HIEs • Comprehensive electronic patient information when and where needed • Allow physicians to have complete and current information upon which to base clinical decisions • Physicians and patients would receive reminders about most recent clinical guidelines and research results What will HIE enable? • Complete medical record always available • Test results and imaging results always available (eliminating repeat tests and imaging) • Decision support always available: guidelines and research results • Real-time aggregation to detect patterns (e.g. bio surveillance) • Quality and payment information derived from record of care – not separate reporting systems • Patients have access to their own records Challenges • • • • • • lack of interoperability lack of defined standards privacy security stakeholder cooperation high costs Elements of HIE • EHR systems – in-patient – out-patient – community practices • Ancillary health care systems – – – – – Pharmacy Laboratory Imaging Physical therapy Home health Elements of HIE • Communication and networking systems – Information moves with patient – Integrated information from all types of providers • Standard medical terminologies – Diagnoses & procedures – Medications – Laboratory test results • Decision support • Privacy and security • Policy development and coordination Common HIE Technical Architecture Models • Centralized Model – patient data is collected from local sources but stored in a central repository – a request for patient data obtains data from the central repository • Decentralized or Federated Model – patient data resides locally in participating organizations – provides a framework for data sharing • Hybrid Model – a cross between a centralized and decentralized architecture – often provides an interface engine through which organizations in the HIE communicate Pennsylvania HIE • Pennsylvania eHealth Partnership Authority was created in July 2012 • The Authority will develop, implement, and maintain: – State-level patient identity management services – State-level patient consent management services (opt-out, opt-back-in) reflecting patient choice – State-level directory of healthcare providers – Certification programs for entities engaging in HIE – Education and awareness materials for consumers and providers about HIE ClinicalConnect • ClinicalConnect is western Pennsylvania’s first HIE • Formed in 2009 as a non-profit organization • Push/pull model: participating organizations pushing continuity of care documents (CCDs), which then get aggregated in ClinicalConnect • Physicians view the information from ClinicalConnect directly via their EHR system • A button within each of the health system’s EHRs launches a query to the HIE to provide a summary view of the patient’s record ClinicalConnect Members ClinicalConnect View of Patient • Physician logs into EHR and selects patient • Physician clicks a link to access HIE data • Patient data is displayed from HIE participants in an integrated view Summary View PCORnet & PaTH Network Funded by PCORI What is PCORI? • The Patient-Centered Outcomes Research Institute (PCORI) was established by the 2010 Patient Protection and Affordable Care Act • PCORI is a non-governmental non-profit institute • PCORI is charged with examining the "relative health outcomes, clinical effectiveness, and appropriateness" of different medical treatments by evaluating existing studies and conducting its own studies What is PCORnet? • PCORnet is the National Patient-Centered Clinical Research Network • A national network for conducting comparative effectiveness research (CER) • Enable a range of observational and experimental CER by establishing a resource of clinical data gathered in healthcare settings, and by patient groups Components of PCORnet • Clinical Data Research Networks (CDRNs) are networks of healthcare systems, such as hospitals and health plans, and collect health information during the routine course of patient care • Patient-Powered Research Networks (PPRNs) are networks operated by groups of patients and their partners, and are focused on a particular condition or population and whose members are interested in sharing health information for research • The Coordinating Center, led by Harvard Pilgrim Health Care Institute and Duke Clinical Research Institute, provides technical and logistical support to the networks 13 CDRNs + 20 PRRNs + 1 CC CDRN Disease Cohorts CDRN Common Cohort Rare Cohort ADVANCE Diabetes Co-infection with HIV and hepatitis C virus CAPriCORN Anemia; Asthma Sickle cell disease; Recurrent C. Difficile colitis Great Plains Collaborative Breast Cancer Amyotrophic Lateral Sclerosis Louisiana Clinical Data Research Network Diabetes Sickle Cell Disease, Rare Cancers NYC-CDRN Diabetes Cystic fibrosis Mid-South CDRN Coronary Heart Disease Sickle Cell Disease PEDSNet Inflammatory bowel disease Hypoplastic left heart syndrome PORTAL Colorectal Cancer Severe Congenital Heart Disease pSCANNER Congestive Heart Failure Kawasaki Disease PaTH Atrial Fibrillation Idiopathic Pulmonary Fibrosis SCIHLS Osteoarthritis Pulmonary arterial hypertension PCORnet Common Cohort • Common Cohort: All CDRNs contribute to a common cohort • Currently, ~85 million patients across all CDRNs • Temple University • UPMC/Pitt • Penn State University • University of Utah • Johns Hopkins University Goals of PaTH • Link EHR data across five diverse healthcare systems so that it can be used to answer questions to improve health and health care, while ensuring patient privacy • Engage patients and clinicians to identify critical research questions for improving health care and to participate in research • Develop convenient survey methods to let patients share their perspectives on health topics with their healthcare teams Moving Data from EHRs to i2b2 ETL: Extract-Transform-Load Cerner PSU i2b2 PSU Heterogeneous Homogenous i2b2 Epic Temple Extract Transform Site specific Little data modification Uniform across PaTH Common Data Elements Load Uniform across PaTH Same i2b2 schema Temple Epic i2b2 JHU JHU Cerner Epic UPMC i2b2 UPMC Use Case: ADAPTABLE Trial • May 2015: First major demonstration of PCORnet for a trial • ADAPATABLE Trial: Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness • Objective: To compare the effectiveness and safety of two doses of aspirin (81 mg and 325 mg) in high-risk patients with coronary artery disease • Primary composite outcome —death, hospitalization for nonfatal myocardial infarction, or stroke • Primary safety end point — major bleeding complications Use Case: ADAPTABLE Trial • Pragmatic clinical trial – Explanatory trials generally measure efficacy - the benefit a treatment produces under ideal conditions, often using carefully defined subjects – Pragmatic trials measure effectiveness-the benefit the treatment produces in routine clinical practice • Embeds the trial within usual care • Recruits a diverse patient population with minimal eligibility criteria • Relies on electronic data collection with reduced need for costly primary data collection • Will recruit 20,000 patients with heart disease • Expected to cost less than $1000 per participant, an amount far below that of a typical trial of this scope ADAPTABLE Protocol Screening of CDRN EHR data with computable phenotype Electronic outreach to potential patients with trial introduction and link to ADAPTABLE web portal • • Web-Based, Electronic Informed Consent Initial patient contact via web portal text and video consent options Developing a common consent form with selected local adaptations Focused questions to confirm patient comprehension for informed consent and eligibility for randomization after consent Randomization and Aspirin dose assignment ACT Network Funded by NCATS/NIH ACT Network Vision • Create a federated research data network for the Accrual of patients for Clinical Trials • Stage 1: Build network for identifying patients using EHR data in real time – Cohort Discovery • Stage 2: Contact and enroll identified patients into clinical trials – Recruitment • Stage 3: Patients and practitioners identify clinical trials – Participation Stage 1: Cohort Discovery • Time frame: 1 year (September 2014 – August 2015) • Number of CTSA sites: 13 in Wave 1 + 8 in Wave 2 = 21 sites • Goal: Create a i2b2/SHRINE network that allows investigators to query EHR data on a limited set of data domains across all sites in real time 21 ACT Sites 11 19 7 4 10 13 17 12 2 20 8 3 1 5 1514 16 6 9 21 18 Legend Red: Wave 1 Blue: Wave 2 ACT Work Structure Executive Committee 4 PIs (Steven Reis, Lee Nadler, Robert Toto, Gary Firestein) plus Work Group Leads Central PM Vince D’Itri (Aspen Advisors) Governance Regulatory Technology Dipti Ranganathan Robert Toto Karen Allen Doug McFadden Nick Anderson Work Groups Data Harmonization Shyam Visweswaran Michael J. Becich Work Groups • Governance Work Group: – Developed Governance Structure Document – Terms of Data-Access Agreement • Regulatory Work Group: – Developed Regulatory Guidance Document – Developed IRB Application Technology Work Group • Technology Work Group: – Helped install i2b2 / SHRINE software at all sites – Installed SHRINE hub at Harvard – Helped troubleshoot network connectivity • Data Harmonization Work Group: – Selected data domains and identified data elements for the ACT Data Model – Selected terminologies and classification systems ACT Data Model • Demographics – birth date, sex, Hispanic status, race, vital status, death date • Diagnoses – diagnosis (ICD-9), diagnosis date, diagnosis source (admit, discharge), diagnosis priority (primary, secondary) • Procedures – procedure (ICD-9), procedure date • Visit Details – admit date, discharge date, visit type (inpatient, ambulatory, ED) • Medications – medication (RxNorm + NDFRT), order date, order type (inpatient, ambulatory) • Laboratory Test Results – lab test (LOINC + LOINC parts hierarchy), specimen date, result location (lab, point of care), result, units Medications NDF-RT classification RxNorm IN (Ingredient) RxNorm SCDF (Ingredient plus dose form) RxNorm SCD and SBD (Ingredient plus strength and dose form) Laboratory Tests LOINC parts hierarchy (LP code) LOINC code Use Case: Rheumatoid Arthritis • Goal: To identify patients with moderate to severe rheumatoid arthritis (RA) and an inadequate response to methotrexate. • Inclusion Criteria: – – – – – – Diagnosis: Rheumatoid arthritis (ICD-9 714.0) Duration of disease: <2 years Active disease: CRP>1.2x ULN or ESR>30 mm/hr Age: Between 18 and 75 years Sex: No criteria Medications: • Methotrexate >3 months at >7.5 mg/week • And currently either on Prednisone dose ≤10 mg/day or not on prednisone • And no current biologic (etanercept, golimumab, adalumimab, anakinra, infliximab, certulizumab, , rituximab) or JAK inhibitor (tofacitinib) Rheumatoid Arthritis • Inclusion Criteria contd.: – Laboratory: • Hgb >10 g/dl, ALT and AST <ULN • And T bili <ULN, Creatinine <ULN • Exclusion Criteria: – – – – – – Active tuberculosis Hepatitis B Hepatitis C HIV Pregnancy Enrolled in another clinical trial Summary & Plans • Summary: – Number of patients in ACT network = ~40 M – Number of patients satisfying RA query = 7,223 from 16/22 sites – Time for RA query = 124 seconds • Plans: – – – – Data characterization Open the network incrementally to investigators Expand ACT to include all 62 CTSA sites Add more data domains ACT Demo • ACT i2b2: http://dbmi-ncatsprod01.dbmi.pitt.edu/webclient/ • ACT SHRINE: http://dbmi-ncatsprod01.dbmi.pitt.edu:6060/shrine-webclient/ Questions? Thank you