* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ISBT 128
Blood sugar level wikipedia , lookup
Schmerber v. California wikipedia , lookup
Autotransfusion wikipedia , lookup
Blood transfusion wikipedia , lookup
Hemorheology wikipedia , lookup
Jehovah's Witnesses and blood transfusions wikipedia , lookup
Blood donation wikipedia , lookup
Rh blood group system wikipedia , lookup
Plateletpheresis wikipedia , lookup
Men who have sex with men blood donor controversy wikipedia , lookup
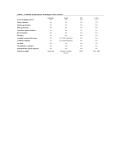
Feature ISBT 128: Beyond the Label Marianne A. Silva, MS, MT(ASCP)SBB, CQA(ASQ) (AABB Consulting Services Division, Bethesda, MD) DOI: 10.1309/HDFCMF4CXD8H8PKV labmedicine.com April 2007 䊏 Volume 38 Number 4 䊏 LABMEDICINE 213 Feature lood suppliers in the United States are in the process of implementing ISBT 128. Many have initiated communication with hospital transfusion services in preparation for this transition. Most facilities use one or more bar codes on the blood bag label to identify, inventory, distribute, or administer the blood component. Changes in labeling will require active communication with many areas of the hospital as information from the blood bag label must be capable of being read, transmitted, translated, printed, and retained in a manner that maintains traceability and unique identification of the component. The transition to ISBT 128 will require evaluation of processes and coordination of multiple departments in each facility. B Beyond Codabar: Why the Transition to ISBT 128 Every label communicates important information to the consumer. While it is common to expect certain types of information on every food or drug label, the same expectation holds true for the label of blood components. The Food and Drug Administration (FDA) specifies in the Code of Federal Regulations1 the information to be included on the label of all blood components. The format or placement of specific information has been consistent for years, based on the “Guidelines for the Uniform Labeling of Blood and Blood Components.”2 This document defined the requirement for the font size, color, and the location of information included on the label of the blood bag. The bar code symbology, Codabar, also has been used for many years by industries worldwide. During the past 20 years, however, as technology has expanded, Codabar has reached the limit of its ability to communicate critical information. It is not able to provide the depth of detail in the product code to adequately describe the donation type, component collected, and modifications made to the component. Additionally, blood centers have found that with Codabar, donor unit numbers may need to be “repeated” within a 10-year period. This can become problematic because a unit of red blood cells can be frozen for that duration of time, raising the potential for a duplicate donation identification number in a facility’s blood inventory. Some facilities also have experienced situations where scanning the bar code of a Codabar label has led to misread or misinterpreted information.3 The complexity of manipulations that can be made to any blood component today demands a robust system for labeling components, and the ability to electronically verify that an appropriate label has been applied to a component provides tremendous value in support of patient safety. Conversion to ISBT 128 bar code symbology will support the advances that have occurred in transfusion medicine and provide a method to communicate critical information. Implementation of ISBT 128 is not mandated by the FDA, but it does fulfill a requirement that specific information on any blood component be “machine readable” by April 24, 2006. This requirement was part of a final rule, the “Bar Code Label Requirement for Human Drug Products and Biological Products,”4 published in the Federal Register (Feb. 26, 2004). The elements that must be machine readable, according to the final rule, include the donation identification number, the ABO/Rh, the product code, and unique facility identifier. 214 LABMEDICINE 䊏 Volume 38 Number 4 䊏 April 2007 Beyond the United States Growth in the fields of transfusion medicine and cellular therapies demands a universal standard specifying a globallyunique donation identification number, an international product reference database, and a standard layout for the product label. ISBT 128 symbology allows a unit to be identified around the world without relying on eye-readable information that may be in a different language to communicate critical data to the end user. This becomes increasingly important when considering international patient and donor databases, military operations, and multinational disaster relief programs. The assignment of a unique facility identification code and the maintenance of the product database is coordinated by ICCBBA, formerly known as the International Council for Commonality in Blood Bank Automation,5 the organization maintaining ISBT 128 and the international information standard. It sets a global standard for the identification, labeling, and information processing of human blood and tissue. Beyond the Blood Bank Implementation of ISBT 128 will not only affect individuals within the blood bank, as other areas of the laboratory may be integrally involved in performing testing on a blood component. For example, the microbiology department may be involved in the evaluation of a transfusion reaction requiring documentation of the donation identification number and component. The hematology department may be involved in the evaluation of a platelet count or white count on a specific blood component, or it may perform testing for hemoglobin S. The chemistry department may be involved in viral marker testing. Anyone involved in recording a donation identification number, whether by electronic or manual means, will need to become familiar with the changes in the blood bag label that will occur with the transition to ISBT 128 as well. Facilities using electronic scanners or interfaces with laboratory equipment will need to evaluate their ability to read and appropriately transmit the information to work lists as well as to a final report. Facilities relying on manual documentation will need to evaluate current documents and forms and ensure that adequate space is available to record the new 13-character donation identification number. Staff will benefit from understanding the structure of the donation identification number (Figure 1). The first 5 characters consist of 1 letter followed by 4 numbers. This is the unique identification number assigned to all facilities worldwide registered with ICCBBA. Facilities within the United States will begin with the letter “W” followed by 4 digits. The following 2 digits represent the year of collection. Unit identification labels that include “07” may be used between December 1, 2006, and January 31, 2008. The inclusion of the year in the donation identification number is to ensure a unique number every 100 years and is not intended to establish a collection or expiration date. Following the year is the 6-digit sequential number representing the donation. The perpendicular numbers are flag characters incorporated into the bar code. These will be read by equipment during scanning. The final character is a manual check character that assists in verifying that the 13-digit donation identification number has been entered correctly when entered manually (using a keyboard) into a computer system. It is not a part of the actual donation identification number. labmedicine.com Feature Figure 1_ISBT 128 13-character donation identification number. Beyond the Laboratory Even beyond the laboratory, there are many departments involved in the administration of blood components within a hospital that will need to become aware of the changes inherent in the transition to ISBT 128. The development of a crossfunctional team with representatives from nursing, anesthesiology, information systems, billing, and medical records may be indicated. They will need to evaluate and revise forms and computer or electronic records to ensure that the 13-character donation identification number can be read, transmitted, printed, and retained appropriately. All health care professionals will need to become familiar with the change in appearance of both allogeneic and autologous blood components (Figures 1 and 2). Currently, the label of the blood bag may have a series of colored tags, stickers, and other labels. Implementation of ISBT 128 will obviate the need for many of those additions to the blood bag label as the information will be incorporated into the bar code. Beyond the Basic Implementation Plan The best way to assess the operational impact of ISBT 128 implementation is to develop a plan that identifies the scope of services provided by a facility. This will determine whether the facility needs to register with ICCBBA. Registration with ICCBBA is different from registration with the FDA, and the criteria defining who must register is not the same (Table 1). The FDA registration (completion of Form FDA 2830 on an annual basis) is required of facilities involved in blood collection and modification of blood components to include irradiation, leukoreduction, washing, freezing, or deglycerolization. Facilities that only pool or aliquot blood components do not need to register with the FDA, but they must register with ICCBBA. Registration with ICCBBA is required for all facilities involved in blood collection and component modification as well as those facilities involved in pooling or the preparation of aliquots, because the label applied to the final component will need to labmedicine.com Figure 2_ISBT 128 autologous label. include ISBT data structures. It is also required for any facility applying a label that uses ISBT 128 data structures. Pooling components, whether platelets or cryoprecipitate, will require the application of a new unit number to the pool. The preparation of an aliquot will require that the product name be changed to reflect that the unit has been divided. This is a change for most who have been involved in transfusion medicine because, with Codabar, an aliquot was represented by adding a suffix to the donation identification number (eg, J12345 is divided into three aliquots represented by J12345A, J12345B, and J12345C). With ISBT 128, when a unit is divided into aliquots, the donation identification number remains the same (J12345), but the product code is changed to reflect that the product is now a divided unit (A0, B0, C0) (Figure 3). Subsequent aliquots— “syringe aliquots”—prepared from the divided unit A0 will be labeled Aa, Ab, etc. Table 1_Registration With ICCBBA Versus Registration With the FDA Services Provided Register With ICCBBA Register With FDA Distribution of blood components only Compatibility testing, distribution Perform any of the following: • Pool platelets • Pool cryoprecipitate • Aliquot components Perform any of the following component modifications: • Irradiate • Leukoreduce • Wash • Freeze • Deglycerolize Collect autologous or allogeneic blood No No Yes No No No Yes Yes Yes Yes April 2007 䊏 Volume 38 Number 4 䊏 LABMEDICINE 215 Feature Conclusion Figure 3_Example of a unit that is divided into three, with two syringe aliquots prepared from aliquot A0 and one syringe aliquot prepared from C0. Once the original unit “00” is divided into three portions, the original “00” no longer exists, but is identified as the divided units A0, B0, and C0. Facilities that divide red cell components into aliquots must also request a variance from the FDA. This applies to registered and non-registered facilities. A variance must be requested because the Code of Federal Regulations requires that for wholeblood and red-cell-containing components, the name of the component must have the anticoagulant preceding the component name.6 This is not required for other divided components. If whole-blood or red-cell-containing aliquots are prepared, a letter of request for variance7 from the Code of Federal Regulations6 must be submitted to the FDA, since implementation of ISBT 128 will not include the name of the anticoagulant preceding the name of the product. 216 LABMEDICINE 䊏 Volume 38 Number 4 䊏 April 2007 During collection, processing, and administration of components, the health care community takes great care to review the detailed information on the blood bag labels. The appearance of the label has to change in order to ensure that each donation includes a globally-unique donation identification number and to ensure there is an adequate description of the contents of the blood bag. With implementation of ISBT 128, the information that must be verified prior to transfusion will be located in a different area of the bag. The coordination of efforts of people beyond the blood bank and processes that extend beyond the laboratory will help drive the success of ISBT 128 implementation efforts. LM Acknowledgment: Thanks to Pat Distler, MS, MT(ASCP) SBB, Technical Director of ICCBBA, who provided the examples of the blood bag labels as well as a technical review of this article prior to submission. Note: ISBT 128 is copyright-protected by United States law and is not in the public domain. Information on how to register is available at www.iccbba.org. 1. 21 CFR 606.121 2. “Guidelines for the Uniform Labeling of Blood and Blood Components” prepared by Food and Drug Administration. Center for Biologics Evaluation and Research, 1985. 3. Silva MA, Shadler A, Figueroa PI, Unit identification error in spite of the use of bar code technology. Transfusion. 2005;45:86A 4. “Bar Code Label Requirement for Human Drug Products and Biological Products” Final Rule. February 26, 2004. 5. www.iccbba.org. 6. 21 CFR 606.121 (e)(1)(ii) and 21 CFR 606.121 (e)(2)(i). 7. 21 CFR 640.120. labmedicine.com