* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atmospheric CO2 targets for ocean acidification perturbation
Survey
Document related concepts
Climate change in Tuvalu wikipedia , lookup
Citizens' Climate Lobby wikipedia , lookup
Economics of global warming wikipedia , lookup
Effects of global warming on human health wikipedia , lookup
Global warming wikipedia , lookup
Politics of global warming wikipedia , lookup
Hotspot Ecosystem Research and Man's Impact On European Seas wikipedia , lookup
Solar radiation management wikipedia , lookup
Climate change and poverty wikipedia , lookup
Instrumental temperature record wikipedia , lookup
Climate change feedback wikipedia , lookup
United Nations Climate Change conference wikipedia , lookup
General circulation model wikipedia , lookup
Iron fertilization wikipedia , lookup
Transcript
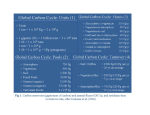
Part 2: Experimental design of perturbation experiments 3 Atmospheric CO2 targets for ocean acidification perturbation experiments James P. Barry1, Toby Tyrrell2, Lina Hansson3,4, Gian-Kasper Plattner5 and Jean-Pierre Gattuso3, 4 Monterey Bay Aquarium Research Institute, USA National Oceanography Centre, University of Southampton, UK 3 Laboratoire d’Océanographie, CNRS, France 4 Observatoire Océanologique, Université Pierre et Marie Curie-Paris 6, France 5 Climate and Environmental Physics, University of Bern, Switzerland 1 2 3.1 Introduction Research on ocean acidification has a primary goal of advancing our understanding of the consequences for marine organisms and ecosystems of future changes in ocean chemistry caused by the anthropogenic rise in atmospheric carbon dioxide levels. Though interesting as a basic research theme, ocean acidification science should play a key role in the development of national and international policies for reducing CO2 emissions. To communicate the science of ocean acidification effectively to industrial leaders, the public and policy makers, the science community must present the results and research implications in clear and consistent terms that relate directly, if possible, to terms used currently in climate discussions, such as atmospheric CO2 levels and potential stabilisation targets. To this end, ocean acidification research programmes should be considered, designed, and reported in the context of realistic ranges for atmospheric p(CO2) levels. Throughout the brief history of ocean acidification research, a spectrum of p(CO2) levels has been used for CO2 perturbation experiments. Though most studies involve the classic levels of ambient (~380 ppm) and future atmospheric p(CO2) (usually 750 ppm, corresponding to the p(CO2) level expected by 2100), values as low as 45 ppm (Buitenhuis et al., 1999) and as high as 150,000 ppm (Kikkawa et al., 2003) have been reported. As long as the scientific community continues to work at different levels of p(CO2), it will be difficult to integrate this information and develop coherent recommendations for policymakers concerning the impacts of fossil fuel emissions on the oceans. To promote direct comparability among studies and provide a clear link to atmospheric targets relevant to climate policy development, it is advantageous to select key values of atmospheric p(CO2) for use as primary targets in ocean acidification experiments. The range of these key values should overlap and span the range of present and future atmospheric levels. Although inclusion of a much broader range of atmospheric p(CO2) levels may be required for various reasons, the greatest societal benefit from ocean acidification studies is likely to lie in increasing our understanding of the functional responses of species, populations, and ecosystems to changes in ocean chemistry that could possibly occur over the next centuries. Unlike atmospheric p(CO2), which is relatively homogeneous over the Earth, aqueous p(CO2) and other ocean carbonate system parameters can vary greatly over space and time (Figure 3.1; Table 3.1). Water temperature and salinity influence the solubility of carbon dioxide in seawater, widening the range of variability in the carbonate chemistry of the oceans, particularly with latitude (Figure 3.2). In deep waters, the accumulation of respiratory carbon dioxide increases the pools of total dissolved inorganic carbon (DIC1) and p(CO2), leading to lower pH, lower carbonate ion (CO 2− 3 ) levels, and reduced saturation states for calcite (Ωc) and aragonite (Ωa). Consequently, pH typically decreases with depth over much of the world ocean, particularly in oxygen minimum zones. In the Eastern Pacific, low-pH waters from oxygen minimum zones upwell over the continental shelf (Feely et al., 2008). € 1 Chapter 1 provides detailed information on the chemical terms and symbols used in the present chapter. Guide to best practices for ocean acidification research and data reporting Edited by U. Riebesell, V. J. Fabry, L. Hansson and J.-P. Gattuso. 2010, Luxembourg: Publications Office of the European Union. 53 Part 2: Experimental design of perturbation experiments Figure 3.1 Carbon dioxide concentrations over the ocean. A. Atmospheric p(CO2) levels (ppm). B. Surface p(CO2) (µatm). Note the change in scale among plots. Data from Takahashi et al. (2009). Temporal variation in carbonate system parameters can also be large, over scales as short as diel cycles (Bensoussan & Gattuso, 2007; Wootton et al., 2008), over seasons (Kleypas et al., 2006; Findlay et al., 2008), and even in association with episodic ENSO events (Friederich et al., 2002). As the balance between photosynthesis and respiration varies over diurnal, seasonal, or other periods, there is a corresponding variation in DIC, pH, and other ocean carbonate system parameters. DIC removal into phytoplankton blooms leads to an elevation of pH, [CO 2− 3 ], Ωc and Ωa during late spring and summer. This is seen in model results and also in data from the Norwegian Sea and in the seas to the north and west of Iceland (Findlay et al., 2008). Distinct, regular and repeated seasonal cycles are also seen at sites closer to the equator, for instance at BATS and HOT (Kleypas et al., 2006). The use of a standard € set of p(CO2)atm targets, converted to p(CO2)aq or other carbonate system parameters in the habitat of concern (e.g. ΩA in tropical surface waters or pH of temperate abyssal depths) will allow reporting of the relevant in situ carbonate system parameters while maintaining a link to the common currency and units of climate change policy. 54 Mean (present-day) Observed range (present-day) Units [DIC] 2020 1840 to 2200 µmol kg-1 strong variation with latitude [AT] 2310 2170 to 2460 µmol kg-1 moderate spatial variability [CO 2− 3 ] 203 81 to 302 µmol kg-1 strong variation with latitude Ωc 4.9 2.0 to 9.1 strong variation with latitude Ωa 3.2 1.2 to 5.4 strong variation with latitude pH 8.1 7.9 to 8.7 strong spatial variability p(CO2) 333 127 to 524 [Ca2+] Parameter € Notes µatm strong spatial variation 10600 µmol kg-1 little spatial or seasonal variation [Mg2+] 55000 µmol kg-1 little spatial or seasonal variation Temp. 18.6 -1.9 to 29.6 °C Salinity 34.8 10.8 to 37.5 - [PO 3-4 ] 0.53 0.01 to 2.11 µmol kg-1 strong variation with latitude [SiO2] 7.35 0.37 to 101 µmol kg-1 high in Southern Ocean strong variation with latitude moderate spatial variation € Table 3.1 Mean and range of variation in the main ocean carbonate system parameters over open ocean surface waters of the world. This table can be used as a reference in the design of experiments including near present-day (1990’s) carbonate system values (future conditions will obviously differ for some parameters). The ranges of total dissolved inorganic carbon (DIC) and AT (total alkalinity) are from the GLODAP database (Key et al., 2004) and represent surface water (0 m) of the open ocean, i.e. excluding coastal, shelf, and enclosed seas, near-shore, and estuarine environments. Other carbonate system parameters were calculated from DIC and AT using the csys software (see main text); temperature (Stephens et al., 2001) and salinity (Boyer et al., 2001) were taken from the World Ocean Atlas (Antonov et al., 2006; Garcia et al., 2006; Locarnini et al., 2006) database. The goal of this chapter is to provide an overview of factors that influence the choice of atmospheric CO2 levels used in ocean acidification studies, based on experimental design, target environments, location, and analytical approach. The overriding philosophy for these guidelines is that ocean acidification research should attempt to provide predictive capabilities concerning the response of the oceans, including its physics, biochemistry, and biology, to a realistic range of future atmospheric p(CO2) levels. 3.2 Approaches and methodologies We investigate the issue of target levels of atmospheric p(CO2), discuss the conversion of atmospheric values into equivalent parameters of ocean carbonate chemistry, and then provide recommendations depending on the number of treatment levels that can be manipulated. 55 Part 2: Experimental design of perturbation experiments Figure 3.2 Distribution of ocean carbonate system parameters over the world ocean during the 1990’s. Total dissolved inorganic carbon (DIC) and AT (total alkalinity) are from the GLODAP database (Key et al., 2004), and represent surface water (0 m) of the open ocean. Other carbonate system parameters were calculated from DIC and AT using the csys software (see main text) and temperature (Stephens et al., 2001) and salinity (Boyer et al., 2001) from the World Ocean Atlas database. Plots courtesy of Andrew Yool. 3.2.1 Selection of key p(CO2)atm values Key values for p(CO2)atm used in ocean acidification experiments should be based mainly on reasonable future trajectories of atmospheric p(CO2), including intermediate stabilisation targets. The Intergovernmental Panel on Climate Change (IPCC) in the Special Report on Emissions Scenarios (SRES; Nakićenović & Swart, 2000) 56 Figure 3.3 Atmospheric p(CO2) levels from Antarctic ice cores at the Law Dome (Etheridge et al., 1998), instrumental observations at Mauna Loa (Keeling et al., 2008), and p(CO2) concentrations resulting from SRES climate scenarios using the Bern carbon cycle model (Nakićenović & Swart, 2000). outlined scenarios projecting fossil fuel CO2 emissions through 2100, based on population and economic growth, rates of technology development, and societal attitudes. SRES scenarios indicate a continual rise in p(CO2)atm with levels at 2100 ranging between ~530 to 970 ppm p(CO2)atm (Figure 3.3), depending on the scenario and carbon cycle model used. Recent models indicate that continued warming of the sea surface will inhibit ocean carbon uptake, leading to ~4% higher atmospheric carbon dioxide levels in 2100 than expected by the SRES scenarios (Plattner et al., 2001). Atmospheric CO2 trajectories beyond 2100 may exceed 1000 ppm, or could approach stabilisation targets from 450 to as high as 1000 ppm, roughly along WRE stabilisation pathways (Wigley et al., 1996). Environmental variability during the recent evolutionary history of marine ecosystems is also important to consider for ocean acidification studies, due to its role in shaping the physiological tolerances of marine organisms and maintaining genetic diversity within populations. Atmospheric CO2 has varied greatly over Earth history (Kasting, 1993), and generally decreased through the Phanerozoic from over 5000 ppm to relatively stable and low levels well below 1000 ppm through most of the Cenozoic (Berner, 1990). Through at least the latter half of the Quaternary (~0.8 My), atmospheric CO2 has generally oscillated rhythmically between glacial (~180 ppm) to interglacial (~280 ppm) extremes (Figure 3.4). This pendulum of p(CO2)atm has driven a parallel modulation of ocean pH, temperature, hypoxia, and other factors, that undoubtedly influenced the recent evolution of a many marine organism. Variation in ocean chemistry during this period is therefore an important context for considering the impacts of future climate scenarios. Although few experimental studies have included p(CO2)atm levels below ambient, Riebesell et al. (2000) included a “glacial” climate (190 ppm CO2) along with present-day (350 ppm) and future (750 ppm) levels in a mescosm study concerning their effects on phytoplankton communities. 57 Part 2: Experimental design of perturbation experiments Figure 3.4 Quaternary atmospheric carbon dioxide (a) based on ice core records (Petit et al. 2001) and estimated ocean pH (b) (J. Barry, unpubl.). Levels of p(CO2)atm exceeding those expected under future climate scenarios may also be useful, for example in studies examining the physiological response of organisms to environmental hypercapnia. Variation in ocean p(CO2), pH and other key parameters can act directly on the physiological performance of organisms, with impacts on the growth, reproduction, behaviour and survival of individuals, which in turn affect the demographic rates (i.e. birth and death rates) of populations, interactions between species and, ultimately, the structure and function of ecosystems. While the objectives of an ocean acidification research project include characterisation of the performance of organisms over a realistic range of future p(CO2)atm levels, inclusion of considerably higher p(CO2) treatments often help constrain their boundaries of performance (e.g. Kurihara & Shirayama, 2004). Knowledge of performance boundaries can also guide subsequent studies on similar taxa. This approach may be particularly important for studies where logistical constraints limit replication or variation among replicates reduces statistical power. Key p(CO2)atm values could be organised according to arbitrary schemes, such as multiples of preindustrial atmospheric levels (PAL) or a range of near log-ratio values (Table 3.2). Multiples of PAL allow 3 or 4 treatment levels in the realm of realistic changes in p(CO2)atm over the next millennium, and relate more closely to the much larger changes in p(CO2)atm levels that have occurred through Earth history (e.g. Kasting, 1993). Other schemes, such as a ~log-ratio method shown in Table 3.2 could span even larger ranges, but provide even fewer treatment levels within reasonable future climate conditions. The range of key values using the latter examples overlaps only marginally with the range of probable future p(CO2)atm levels, reducing their relevance for the general goals of ocean acidification research. Refer to chapter 4 for further information on statistics. 58 Table 3.2 Alternative designs for key atmospheric p(CO2) values used in ocean acidification studies. • Realistic range of atmospheric p(CO2) levels including glacial, preindustrial (280 ppm) and intermediate values to 2100 and beyond (1000 ppm; Wigley, 1996; Nakićenović & Swart 2000): 180, 280, 380, 450, 550, 650, 750, 1000 • Integer multiples of 280 ppm (pre-industrial value): 280, 560, 840, 1120, 1400, 1680, 1960, 2240, 2520, 2800, 4480, … • Range of ~log-ratio values to investigate the response to exposure to very high p(CO2)atm levels: 80, 1000, 3000, 10000, 30000, 100000, ... 3.2.2 Conversion of atmospheric p(CO2) levels to corresponding in situ ocean chemistry Key atmospheric p(CO2) values can be defined and used as guidelines, but their corresponding values for ocean carbonate system parameters are the primary measurements for ocean acidification experiments, and should also be reported. How can investigators convert key atmospheric p(CO2) values to the in situ p(CO2), pH, Ωa, Ωc, or other carbonate system parameters of interest for specific ocean acidification experiments? The simplest method may be to use published predictions of future ocean carbonate system values, based on changes in atmospheric p(CO2) (Gattuso & Lavigne, 2009). Examples include predictions of future changes in ocean pH and carbonate saturation states (Gattuso et al., 1999, Caldeira & Wickett, 2003, 2005; Guinotte et al., 2003; Feely et al., 2004; Orr et al., 2005; Kleypas et al., 2006; Hoegh-Guldberg et al., 2007). It is worth noting that the present-day latitudinal trends in some carbonate system variables will exist in the future, as seen for example in Figure 3.5 (Orr et al., 2005). If all recoverable fossil fuels are eventually burnt, pH is expected to decline by about 0.8 units from the pre-industrial total, leading to an average surface pH decline from about 8.2 down to 7.4 (Caldeira & Wickett, 2003). An alternate approach for predicting future ocean carbonate system values is to assume that surface water is in equilibrium with the atmosphere and use software such as co2sys (http://cdiac.ornl.gov/oceans/co2rprt.html), seacarb (http://www.obs-vlfr.fr/~gattuso/seacarb.phphttp://cran.at.r-project.org/web/packages/seacarb/), or csys (http://www.soest.hawaii.edu/oceanography/faculty/zeebe_files/CO2_System_in_Seawater/csys.html). Each of these software packages can calculate a series of ocean carbonate parameters based on input for other carbonate system factors (see chapter 2 for examples of calculations using seacarb). Knowledge of 2 of 4 key carbonate parameters (p(CO2), pH, DIC, AT) and a few physical factors (temperature, salinity, [SiO2], [ PO 3− 4 ], depth) is sufficient to calculate all other carbonate system values (see Dickson, this volume, for detailed information). This approach is particularly important for locations differing from the open ocean, such as coastal zones, inland seas, oxygen minimum zones, and deep-sea environments. Large divergence of the concentrations of calcium [Ca2+] and/ € or magnesium [Mg2+] from normal seawater values affects the calculation of calcite saturation state from [CO 2− 3 ] (Tyrrell & Zeebe, 2004). For some habitats and microenvironments, p(CO2)atm can be considerably higher than observed atmospheric levels. € Using carbonate chemistry software, investigators can substitute a future atmospheric carbon dioxide level (e.g. 750 ppm) for p(CO2) in surface waters, then combine this with AT to approximate future DIC, Ωa, Ωc, or pH. Because p(CO2) varies over the world ocean (Figure 3.1), a somewhat more accurate use of this method would use the p(CO2) in surface waters at the location of interest, then increase it by an increment corresponding to the increase in p(CO2)atm of interest (e.g. +365 ppm: the difference between 750 and 385 ppm). Carbonate system parameters could then be calculated assuming no change in AT. For deeper waters, the increase in DIC calculated for the surface could be added to observed deep-water DIC levels, and combined with in situ AT to calculate other carbon system elements. For example, 30°C surface water with a total alkalinity of 2264 µmol kg-1 equilibrated with a 380 ppm atmosphere, has a DIC concentration of ~1919 µmol kg-1 and an Ωa of 3.94, compared to a similar sample at 5°C, in which DIC is 2107 µmol kg-1 and Ωa drops to only 1.74. Conversion 59 Part 2: Experimental design of perturbation experiments of atmospheric targets to deep-sea values can produce even larger changes. Due to low water temperatures and the accumulation of respiratory CO2 at depth, p(CO2)atm values of 380 and 750 ppm can be equivalent to bathyal p(CO2) values of 1000 and >3000 µatm, respectively, particularly in oxygen minimum zones. Atmospheric p(CO2) is typically reported for dry air, and should be adjusted for the vapour pressure of water (100% humidity) near the ocean surface (Zeebe & WolfGladrow, 2001), leading to a decrease in p(CO2) of about 3%. Factors that will affect the predicted carbonate system parameters at key p(CO2)atm values include location, temperature, depth, productivity, regional to local oceanographic dynamics, the stability of the climate system and its recent history. The heterogeneous distribution of carbonate chemistry in the world ocean derives from the dynamic quasiequilibrium that exists currently in the atmosphereocean system. As indicated before, even though p(CO2)atm is relatively homogenous over the globe, p(CO2) in surface waters is highly heterogeneous (Figure 3.1). Estimating accurately the change in p(CO2) at a particular location under an atmospheric Figure 3.5 Predicted changes in future atmospheric level of 750 ppm CO2, particularly in waters deep p(CO2), ocean pH and ocean carbonate ion concentrations. beneath the surface, is not straightforward. The ‘I’ in b and c refers to the IS92a future scenario, ‘S’ to predicted values, particularly for deep-sea waters the S650 scenario. (From Orr et al. (2005). Reprinted by permission from MacMillan Publishers Ltd: Nature, that respond slowly to recent changes in atmospheric Copyright (2005)). CO2, depend upon the stability of the climate and the time scales for equilibration for the atmosphereocean carbonate system. Failures of other assumptions, such as the long-term stability of total alkalinity (e.g. Ilyina et al., 2009), ocean ventilation, carbonate rain ratios, etc. under continued climate change, also affect the accuracy of carbonate system predictions. 3.2.3 The number of treatments in ocean acidification experiments The ideal perturbation experiment would measure the response of the experimental system (e.g. phytoplankton community dynamics, coral calcification or animal physiology) to a range of pH (or other carbonate system parameters) corresponding to a series of atmospheric p(CO2) from 180 to 1000 ppm or higher. This would provide an understanding of the performance of organisms under glacial climate, present-day, and the near future, including the trend over a range in p(CO2)atm as well as inflection points in performance that could indicate tipping points. In practice, however, logistical considerations limit the number of treatment levels and replication, thereby reducing the predictive resolution of the results. How many treatments levels are sufficient? What statistical design is optimal? At a minimum, 2 or 3 treatments levels analysed using an analysis of variance (ANOVA) design can yield valuable comparisons among locations or pH treatments (e.g. Manzello et al., 2008). Analysis of variance is often used to compare differences among categorical variables, and may be the optimal design for studies limited to a few treatments. However, when the number of treatment levels can be increased, regression designs are typically more advantageous, particularly for continuous variables (e.g. ocean pH). 60 Since the parameters and processes measured are continuous variables, regression designs, particularly those with many treatment levels, allow interpretations of results that define functional relationships (e.g. variation in animal performance over a range of p(CO2) levels), and may be more effective in identifying tipping points, if they exist. Although three treatments is a minimum for regression analyses, additional treatments, even at the expense of replication within treatments, often provides greater inferential power for a continuous variable. This approach may be particularly valuable in detecting potential tipping points. Regression may be less effective for systems where within-treatment responses are variable, and analysis of variance designs may increase statistical power. A more thorough discussion of experimental design is presented in chapter 4. Where to start? At a minimum, perturbation experiments typically compare one or more treatments simulating future atmospheric CO2 levels to a baseline control treatment. A “preindustrial” climate near 280 ppm p(CO2)atm is the most suitable baseline treatment, since it represents a long-term (i.e. millennial) average concentration that has shaped animal performance and ecosystem function. Ambient or “present-day” p(CO2)atm (385 ppm CO2 = 2008 average on Mauna Loa; http://cdiac.ornl.gov/ftp/trends/co2/maunaloa.co2) has also been used as a baseline treatment in various studies, for comparing animal performance with future, higher p(CO2)atm levels. However, because the amount of anthropogenic carbon dioxide in the atmosphere has doubled nearly every 31 years (Hofmann et al., 2008), “present-day” is a rapidly shifting target, and has already exceeded a p(CO2)atm threshold (350 ppm) proposed as a potential ecological tipping point (Hansen et al., 2008). Guidelines presented here (Table 3.3) are based on the number of treatment levels that can be supported both technically and financially. For studies limited to very few perturbed treatment levels, comparisons between “presentday” values (currently ~385 ppm), and 750 ppm (“future”) are recommended as primary treatment levels. While “preindustrial” (280 ppm) p(CO2)atm may be more relevant as a control treatment for many organisms than presentday p(CO2)atm, 280 ppm is technically difficult to achieve for most experiments, and “present-day” can be substituted as an unperturbed, control treatment. Ambient p(CO2)atm has value as a p(CO2)atm treatment because natural systems have acclimated to this level over decadal time scales, and because it provides a context for current changes in response variables. For a future treatment, 750 ppm is favoured after considering that ongoing efforts to curb fossil fuel CO2 emissions, recent emission records, and climate modelling based on (Nakićenović & Swart, 2000; Plattner et al., 2001) indicate p(CO2)atm will reach or exceed 750 ppm by 2100, a value midway between SRES scenario A1B and A2 (Figure 3.3). In addition, a 2-treatment comparison between these values is more likely to detect significant changes in performance than between smaller p(CO2)atm changes, particularly for field experiments where withintreatment variability can be high. Inclusion of “preindustrial” p(CO2)atm (280 ppm) conditions should be considered as a third treatment. Using this 2 or 3-treatment design, it should be possible to document changes to date (i.e. since ~1850), and project changes likely to occur by the end of this century. As the number of treatment levels increases, the addition of stabilisation targets and important potential tipping points (e.g. 350 ppm: Hansen et al., 2008; 450 ppm: Hoegh-Guldberg et al., 2007, McNeil & Matear, 2008) for atmospheric CO2 can be added. Tipping points can be crucial for climate policy development and for increased awareness of society to the potentially non-linear response of Earth systems to climate change (Lenton et al., 2008). Key values of atmospheric p(CO2) recommended for ocean acidification studies (Table 3.3) can be used as guidelines for the design of experiments. These guidelines may require modification to fit particular needs, such as the addition of higher values to examine the boundaries of animal performance or to allow closer correspondence with crucial levels for specific carbonate system parameters (e.g. Ωa or Ωc ~1). Increasing the number of treatments will provide greater predictive power, particularly for non-linear responses to ocean acidification. Even if this chapter is devoted to a discussion on atmospheric CO2 targets, it is nevertheless important to note the potential synergy between multiple stressors. The effects of ocean acidification, thermal stress, and expanding hypoxia, all linked to anthropogenic climate change, act together to constrain the window of performance for marine organisms (Pörtner, 2008). Therefore, consideration of multiple climate stressors for ocean acidification experiments will elevate the value of these studies, allowing an integrated view of climate change impacts on ocean ecosystems. 61 Part 2: Experimental design of perturbation experiments Table 3.3 Key p(CO2)atm values (ppm) for ocean acidification studies. These (CO2)atm levels are useful guidelines for perturbation experiments, and can be supplemented with other values of importance for specific studies, such as higher values for evaluating animal performance, or adjustments to correspond to key carbonate system values (e.g. Ωa or Ωc ~1). # of Treatments 3.3 2 present-day (~385), 750 3 280, present-day, 750 4 280, present-day, 550, 750 6 280, present-day, 550, 650, 750, 1000 8 180, 280, present-day, 450, 550, 650, 750, 1000 >8 Add values (e.g. 350, other) to increase resolution Strengths and weaknesses − Designing ocean acidification experiments in the context of realistic ranges in future atmospheric carbon dioxide levels will increase our understanding of the effects of impending environmental change and enable information-based policy development for climate adaptation. − Use of key atmospheric carbon dioxide values as the principal treatment levels for ocean acidification experiments will provide a strong link between ocean acidification science and climate policy discussion. − Comparison of results among ocean acidification studies will be easier by using common atmospheric CO2 targets, even though ocean carbonate chemistry parameters may differ. − Regression designs with higher numbers of ocean acidification treatment levels are, in general, more likely to identify tipping points in organism or community performance than those with fewer treatment levels. 3.4 62 Recommended p(CO2)atm levels Potential pitfalls − Conversion of key atmospheric p(CO2) values to specific parameters of the ocean carbonate system can be complex. − Characterisation of the effects of tipping points will be difficult, even if speculations about such values exist. Tipping points (p(CO2)atm) likely differ from single organisms to whole ecosystems with complex direct and indirect consequences, time scales, and effects. On the other hand, some studies indicate a tipping point of 450 ppm for coral reefs, and if this point is not included, important results might be neglected. − Using too narrow a scope of p(CO2)atm levels under conditions of high within-treatment variability may reduce the statistical power of analyses of variance. − Physiological processes depend strongly on temperature and are often subject to a narrow thermal window (Pörtner et al., 2004). This should not be neglected and multifactor experiments studying the interacting effects of p(CO2)atm, temperature, and perhaps oxygen should, if possible, be conducted. See chapter 9 of this Guide for more information. − Logistical and other constraints limiting the number of p(CO2) treatments can reduce inference concerning non-linear functional responses of organisms to ocean acidification. 3.5 Suggestions for improvements Society and the field of ocean acidification science will benefit from the standardisation of atmospheric carbon dioxide levels as the common currency for discussion of perturbation studies. Use of atmospheric levels will promote the effective communication of results from ocean acidification studies to policymakers, and increase the impact of ocean acidification science in the development of climate adaptation policy. Within the oceanographic community the combined use of atmospheric p(CO2) levels and related ocean carbonate chemistry parameters will help standardise comparisons of the potential effects of ocean acidification among habitats and ecosystems. Increased focus on the characterisation of functional responses through the use of higher numbers of treatment levels will also increase the relevance of ocean acidification studies for society. Higher resolution among treatment levels will help characterise non-linear relationships between p(CO2)atm levels and ecosystem processes, and help evaluate ecosystem function near proposed tipping points. 3.6 Data reporting The primary goal of data reporting for climate targets is to provide a template for comparing experimental results within the ocean science community, as well as between ocean and terrestrial systems. The common currency of atmospheric carbon dioxide levels and the use of standard or key p(CO2)atm values for most studies will elevate the value of ocean acidification science for society. Towards that goal, ocean acidification studies should report carefully the p(CO2)atm levels of interest for the study, with the corresponding levels of the various parameters of the carbonate system. Atmospheric carbon dioxide values should be used in the abstract, discussion, and conclusions of papers to maximise the likelihood that terms common within climate discussions are used clearly. Also required is a careful reporting of relevant ocean carbonate chemistry. While some values (p(CO2)) may be similar to target values, others (e.g. carbonate saturation, bicarbonate levels, etc.) may not. Considering the growing concern for the combined impacts of warming, acidification, and environmental hypoxia, it is advisable to report these and other potentially relevant environmental parameters. 3.7 Recommendations for standards and guidelines 1. Ocean acidification experiments should be based primarily on a broad range of realistic p(CO2)atm values spanning glacial, present-day, and the future (1000+ ppm). 2. Atmospheric p(CO2) values of 180, 280, 350, “present-day”, 450, 550, 650, 750, and 1000 are guidelines for designing ocean acidification experiments. 3. For logistically limited studies, primary targets of 280, present-day (currently 385), and 750 ppm should be used. 4. Values of p(CO2)atm exceeding realistic ranges can be useful to examine the boundaries of animal performance, but should be followed by or coupled with realistic values. 5. Key atmospheric carbon dioxide levels should be converted to corresponding values of in situ ocean carbonate parameters for specific ocean acidification experiments. 6. Key p(CO2)atm and in situ carbonate system variables should be reported in tandem in publications reporting the results of ocean acidification experiments. 7. An increased number of treatments, even with reduced replication, may have greater power to characterise the functional relationship between ocean acidification parameters and organism or ecosystem performance. 8. Use of increased treatment levels increases the likelihood of detecting “tipping points” in organism or community performance. 63 Part 2: Experimental design of perturbation experiments 3.8 References Antonov J. I., Locarnini R. A., Boyer T. P., Mishonov A. V. & Garcia H. E., 2006. World Ocean Atlas 2005. Volume 2: Salinity. In: Levitus S. (Ed.), NOAA Atlas NESDIS 62, pp. 1-182. Washington, D.C.: U.S. Government Printing Office. Bensoussan N. & Gattuso J.-P., 2007. Community primary production and calcification in a NW Mediterranean ecosystem dominated by calcareous macroalgae. Marine Ecology Progress Series 334:37-45. Berner R. A., 1990. Atmospheric carbon dioxide levels over Phanerozoic time. Science 249:1382-1386. Boyer T. P., Stephens C., Antonov J. I., Conkright M. E., Locarnini R. A., O’Brien T. D. & Garcia H. E., 2002. World Ocean Atlas 2001, Volume 2: Salinity. In: Levitus S. (Ed.), NOAA Atlas NESDIS 50, pp. 1-165. Washington D.C.: U.S. Government Printing Office. Buitenhuis E. T., de Baar H. J. W. & Veldhuis M. J. W., 1999. Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species. Journal of Phycology 35:949–959. Caldeira K. & Wickett M., 2003. Anthropogenic carbon and ocean pH. Nature 425:365. Caldeira K. & Wickett M. E., 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. Journal of Geophysical Research- Oceans 110, C09S04. doi:10.1029/2004JC002671. Etheridge D. M., Steele L. P., Langenfelds R. L., Francey R. J., Barnola J.-M., & Morgan V. I., 1998. Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. In Trends: A compendium of data on global change. Oak Ridge, Tenn., U.S.A.: U.S. Department of Energy. Feely R. A., Sabine C. L., Hernandez-Ayon J. M., Ianson D. & Hales B., 2008. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320:1490-1492. Feely R. A., Sabine C. L., Lee K., Berelson W., Kleypas J., Fabry V. J. & Millero F. J., 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305:362-366. Findlay H. S., Tyrrell T., Bellerby R. G. J., Merico A. & Skjelvan I., 2008. Carbon and nutrient mixed layer dynamics in the Norwegian Sea. Biogeosciences 5:1395–1410. Friederich G. E., Walz P. M., Burczynski M. G. & Chavez F. P., 2002. Inorganic carbon in the central California upwelling system during the 1997-1999 El Niño-La Niña event. Progress in Oceanography 54:185-203. Garcia H. E., Locarnini R. A., Boyer T. P. & Antonov J. I., 2006. World Ocean Atlas 2005. Volume 4: Nutrients (phosphate, nitrate, silicate). In: Levitus S. (Ed.), NOAA Atlas NESDIS 64, pp. 1-396. Washington, D.C.: U.S. Government Printing Office. Gattuso J.-P., Allemand D. & Frankignoulle M., 1999. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. American Zoologist 39:160-183. Gattuso J.-P. & Lavigne H., 2009. Technical Note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences 6:2121-2133. Guinotte J. M., Buddemeier R. W. & Kleypas J. A., 2003. Future coral reef habitat marginality: temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 22:551-558. Hansen J., Sato M., Kharecha P., Beerling D., Berner R., Masson-Delmotte V., Pagani M., Raymo M., Royer D. L. & Zachos J. C., 2008. Target atmospheric CO2: Where should humanity aim? The Open Atmospheric Science Journal 2:217-231. Hoegh-Guldberg O., Mumby P. J., Hooten A. J., Steneck R. S., Greenfield P., Gomez E., Harvell C. D., Sale P. F., Edwards A. J., Caldeira K., Knowlton N., Eakin C. M., Iglesias-Prieto R., Muthiga N., Bradbury R. H., Dubi A. & Hatziolos M. E., 2007. Coral reefs under rapid climate change and ocean acidification. Science 318:1737-1742. Hofmann D. J., Butler J. H. & Tans P. P., 2008. A new look at atmospheric carbon dioxide. Atmospheric Environment 43:2084-2086. Ilyina T., Zeebe R. E., Maier-Reimer E. & Heinze C., 2009. Early detection of ocean acidification effects on marine calcification. Global Biogeochemical Cycles 23, GB1008. doi:10.1029/2008GB003278. 64 Kasting J., 1993. Earth’s early atmosphere. Science 259:920-926. Keeling R. F., Piper S. C., Bollenbacher A. F. & Walker J. S., 2008. Atmospheric CO2 records from sites in the SIO air sampling network. In Trends: A compendium of data on global change. Oak Ridge, Tenn.: Oak Ridge National Laboratory, U.S. Department of Energy. Key R. M., Kozyr A., Sabine C. L., Lee K., Wanninkhof R., Bullister J. L., Feely R. A., Millero F. J., Mordy C. & Peng T. H., 2004. A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP). Global Biogeochemical Cycles 18, GB4031. doi:10.1029/2004GB002247. Kikkawa T., Ishimatsu A. & Kita J., 2003. Acute CO2 tolerance during the early developmental stages of four marine teleosts. Environmental Toxicology 18:375-382. Kleypas J. A., Feely R. A., Fabry V. J., Langdon C., Sabine C. L. & Robbins L. L., 2006. Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research. 88 p. Boulder, Colorado: Institute for the Study of Society and Environment (ISSE) of the University Corporation for Atmospheric Research (UCAR). Kurihara H. & Shirayama Y., 2004. Effects of increased atmospheric CO2 on sea urchin early development. Marine Ecology Progress Series 274:161-169. Lenton T. M., Held H., Kriegler E., Hall J. W., Lucht W., Rahmstorf S. & Schellnhuber H. J., 2008. Tipping elements in the Earth’s climate system. Proceedings of the National Academy of Science U.S.A. 105:1786-1793. Locarnini R. A., Mishonov A. V., Antonov J. I., Boyer T. P. & Garcia H. E., 2006. World Ocean Atlas 2005. Volume 1: Temperature. In: Levitus S. (Ed.), NOAA Atlas NESDIS 61, pp. 1-182. Washington, D.C.: U.S. Government Printing Office. Manzello D. P., Kleypas J. A., Budd D. A., Eakin C. M., Glynn P. W. & Langdon C., 2008. Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world. Proceedings of the National Academy of Science U.S.A. 105:10450-10455. McNeil B. I. & Matear R., 2008. Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proceedings of the National Academy of Science U.S.A. 105:18860-18864. Nakićenović N. & Swart R., 2000. Special report on emissions scenarios: a special report of Working Group III of the Intergovernmental Panel on Climate Change, 599 p. Cambridge: Cambridge University Press. Orr J. C., Fabry V. J., Aumont O., Bopp L., Doney S. C., Feely R. A., Gnanadesikan A., Gruber N., Ishida A., Joos F., Key R. M., Lindsay K., Maier-Reimer E., Matear R., Monfray P., Mouchet A., Najjar R. G., Plattner G. K., Rodgers K. B., Sabine C. L., Sarmiento J. L., Schlitzer R., Slater R. D., Totterdell I. J., Weirig M. F., Yamanaka Y. & Yool A., 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681-686. Petit J. R., Jouzel J., Raynaud D., Barkov N. I., Barnola J.-M., Basile I., Bender M., Chappellaz J., Davis J., Delaygue G., Delmotte M., Kotlyakov V. M., Legrand M., Lipenkov V., Lorius C., Pépin L., Ritz C., Saltzman E. & Stievenard M., 2001. Vostok ice core data for 420,000 years. IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series #2001-076: Boulder, CO: NOAA/NGDC Paleoclimatology Program. Plattner G. K., Joos F., Stocker T. F. & Marchal O., 2001. Feedback mechanisms and sensitivities of ocean carbon uptake under global warming. Tellus B 53:564-592. Pörtner H.-O., 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Marine Ecology Progress Series 373:203-217. Pörtner H.-O., Langenbuch M. & Reipschlager A., 2004. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. Journal of Oceanography 60:705-718. Riebesell U., Zondervan I., Rost B., Tortell P. D. & Morel F. M. M., 2000. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364-367. Stephens, C., J. I. Antonov, T. P. Boyer, M. E. Conkright, R. A. Locarnini, T. D. O’Brien, H. E. Garcia, 2002. World Ocean Atlas 2001, Volume 1: Temperature. In: Levitus S. (Ed.), NOAA Atlas NESDIS 49, pp. 1-167. Washington D.C.: U.S. Government Printing Office. 65 Takahashi T., Sutherland S. C., Wanninkhof R., Sweeney C., Feely R. A., Chipman D. W., Hales B., Friederich G., Chavez F., Sabine C., Watson A., Bakker D. C. E., Schuster U., Metzl N., Inoue H. Y., Ishii M., Midorikawa T., Nojiri Y., Körtzinger A., Steinhoff T., Hoppema M., Olafsson J., Arnarson T. S., Tilbrook B., Johannessen T., Olsen A., Bellerby R., Wong C. S., Delille B., Bates N. R. & de Baar H. J. W., 2009. Climatological mean and decadal change in surface ocean pCO2, and net sea-air CO2 flux over the global oceans. Deep-Sea Research II 56:554-577. Tyrrell T. & Zeebe R. E., 2004. History of carbonate ion concentration over the last 100 million years. Geochimica et Cosmochimica Acta 68:3521-3530. Wigley T. M. L., Richels R. & Edmonds J. A., 1996. Economic and environmental choices in the stabilization of atmospheric CO2 concentrations. Nature 379:240-243. Wooton J. T., Pfister C. A. & Forester J. D., 2008. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proceedings of the National Academy of Science U.S.A. 105:18848-18853. Zeebe R. E. & Wolf-Gladrow D. A., 2001. CO2 in seawater: equilibrium, kinetics, isotopes. 346 p. Amsterdam: Elsevier. 66