* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 9: Radiation processes Almost all astronomical information
Metastable inner-shell molecular state wikipedia , lookup
X-ray astronomy detector wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Stellar evolution wikipedia , lookup
Main sequence wikipedia , lookup
Health threat from cosmic rays wikipedia , lookup
Standard solar model wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Star formation wikipedia , lookup
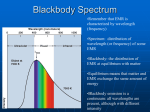
PH507 Astrophysics Dr Dirk Froebrich -1- Lecture 9: Radiation processes Almost all astronomical information from beyond the Solar System comes to us from some form of electromagnetic radiation (EMR). We can now detect and study EMR over a range of wavelength or, equivalently, photon energy, covering a range of at least 1016 - from short wavelength, high photon energy gamma rays to long wavelength low energy radio photons. Out of all this vast range of wavelengths, our eyes are sensitive to a tiny slice of wavelengths- roughly from 4500 to 6500 Å. The range of wavelengths our eyes are sensitive to is called the visible wavelength range. We will define a wavelength region reaching somewhat shorter (to about 3200 Å) to somewhat longer (about 10,000 Å) than the visible as the optical part of the spectrum. Physicists measure optical wavelengths in nanometers (nm). Astronomers tend to use Angstroms. 1 Å = 10-10 m = 0.1 nm. Thus, a physicist would say the optical region extends from 320 to 1000 nm.) All EMR comes in discrete lumps called photons. A photon has a definite energy and frequency or wavelength. The relation between photon energy (Eph) and photon frequency ν is given by: Eph = hν or, since c = λ ν E ph = hc λ where h is Planck’s constant and λ is the wavelength, and c is the speed of light. The energy of visible photons is around a few eV (electron volts). (An electron volt is a non- metric unit of energy that is a good size for measuring energies associated with changes of electron levels in atoms, and also for measuring energy of visible light photons. 1 eV = 1.602 x 10-19 Joules.) An approximate value (1 in 104) for the energy of electromagnetic radiation expressed in electron volts is given by 1234 / λ, where λ is the wavelength in nanometres. PH507 Astrophysics Dr Dirk Froebrich -2- In purely astronomical terms, the optical portion of the spectrum is important because most stars and galaxies emit a significant fraction of their energy in this part of the spectrum. (This is not true for objects significantly colder than stars - e.g. planets, interstellar dust and molecular clouds, which emit in the infrared or at longer wavelengths - or significantly hotter- e.g. ionised gas clouds, neutron stars, which emit in the ultraviolet and x-ray regions of the spectrum. Another reason the optical region is important is that many molecules and atoms have electronic transitions in the optical wavelength region. We will define the regions of the Electromagnetic Spectrum to have wavelengths as follows: • • • • • • Gamma-rays: < 0.1Å, highest frequency, shortest wavelength, highest energy. X-Rays: 0.1Å -- 100Å Ultraviolet light: 100Å -- 3000Å Visible light: 3000Å -- 10000Å = 1µ µm (micrometer or micron) Infrared Light: 1µ µm -- 1mm (NIR, MIR, FIR, sub-mm) Radio waves: >1mm, lowest frequency, longest wavelength, lowest energy. Wavelength Range Radio Infrared Visible Ultraviolet X-rays Gamma rays wavelength > 10-4 m = 1 mm 700 nm < wavelength < 1 mm 400 nm < wavelength < 700 nm 20 nm < wavelength < 400 nm 0.1 nm < wavelength < 20 nm wavelength < 0.1 nm PH507 Astrophysics Dr Dirk Froebrich -3- Blackbody Radiation Where then does a thermal continuous spectrum come from? Such a continuous spectrum comes from a blackbody whose spectrum depends only upon the absolute temperature. A blackbody is so named because it absorbs all electromagnetic energy incident upon it - it is completely black. To be in perfect thermal equilibrium, however, such a body must radiate energy at exactly the same rate that it absorbs energy; otherwise, the body will heat up or cool down (its temperature will change). Ideally, a blackbody is a perfectly insulated enclosure within which radiation has come into thermal equilibrium with the walls of the enclosure. Practically, blackbody radiation may be sampled by observing the enclosure through a tiny pinhole in one of the walls. The gases in the interior of a star are opaque (highly absorbent) to all radiation (otherwise, we would see the stellar interior at some wavelength!); hence, the radiation there is blackbody in character. We sample this radiation as it slowly leaks from the surface of the star - to a rough approximation, the continuum radiation from some stars is blackbody in nature. Planck’s Radiation Law After Maxwell's theory of electromagnetism appeared in 1864, many attempts were made to understand blackbody radiation theoretically. None succeeded until, in 1900, Max K. E. L. Planck (1858-1947) postulated that electromagnetic energy can propagate only in discrete quanta, or photons, each of energy E = hν. He then derived the spectral intensity relationship, or Planck blackbody radiation law: 2hν 3 1 I(ν )dν = c 2 hkTν e − 1 2 where I(v)dν is the intensity (J/m /s/sr) of radiation from a blackbody at temperature T in the frequency range between v and v + dv, h is Planck's constant, c is the speed of light, and k is Boltzmann's constant. Note the exponential in the denominator. PH507 Astrophysics Dr Dirk Froebrich -4- Because the frequency v and wavelength λ of electromagnetic radiation are related by λv = c, we may also express Planck's formula in terms of the intensity emitted per unit wavelength interval: This is illustrated for several values of T: Note that both I(λ) and I(ν) increase as the blackbody temperature increases - the blackbody becomes brighter. This effect is easily interpreted when we note that I(ν)∆ν is directly proportional to the number of photons emitted per second near the energy hν. The Planck function is special enough so that its given its own symbol, B(λ) or B(ν), for intensity. Long wavelengths: Rayleigh tail : B(λ ) ∝ 1 / λ4 Wien’s Law A blackbody emits at a peak intensity that shifts to shorter wavelengths as its temperature increases. PH507 Astrophysics Dr Dirk Froebrich -5- • Wilhelm Wien (1864-1928) expressed the wavelength at which the maximum intensity of blackbody radiation is emitted - the peak (that wavelength for which dI(λ)/d λ = 0) of the Planck curve (found from taking the first derivative of Planck's law) - by Wien's displacement law: λ max = 2.898 x 10-3 / T where λ max is in metres when T is in Kelvin. Note that because λ maxT = constant, increasing one proportionally decreases the other. For example, the continuum spectrum from our Sun is approximately blackbody, peaking at λ max ≈ 500nm. Therefore, the surface temperature is near 5800 K. PH507 Astrophysics Dr Dirk Froebrich -6- The Law of Stefan and Boltzmann The area under the Planck curve (integrating the Planck function) represents the total energy flux, F (W/m2), emitted by a blackbody when we sum over all wavelengths and solid angles: The formula is: • The constant "sigma" is called the Stefan-Boltzmann constant and is given by: • The temperature in this equation is the surface temperature of the object! The object might be much hotter deep inside, but this doesn't matter. PH507 Astrophysics Dr Dirk Froebrich -7- The strong temperature dependence of this formula was first deduced from thermodynamics in 1879 by Josef Stefan (1835-1893) and was derived from statistical mechanics in 1884 by Boltzmann. Therefore we call the expression the Stefan-Boltzmann law. The brightness of a blackbody increases as the fourth power of its temperature. If we approximate a star by a blackbody, the total energy output per unit time of the star (its power or luminosity in watts) is just L = 4πR2σT4 since the surface area of a sphere of radius R is 4πR2 To summarise: A blackbody radiator has a number of special characteristics. One, a blackbody emits some energy at all wavelengths. Two, a hotter blackbody emits more energy per unit area and time at all wavelengths than does a cooler one. Three, a hotter blackbody emits a greater proportion of its radiation at shorter wavelengths than does a cooler one. Four, the amount of radiation emitted per second by a unit surface area of a blackbody depends on the fourth power of its temperature. PH507 Astrophysics Dr Dirk Froebrich -8- Stellar Material Our Sun is the only star for which I(λ) has been accurately observed. Indeed, Lbol is related to the solar constant: the total solar radiative flux received at the Earth’s orbit outside our atmosphere (1370 W/m2). The solar luminosity Lu (3.86 x 1026 W) is calculated from the solar constant in the following manner. Using the inverse-square law, we find the radiative flux at the Sun’s surface R. Then L .is just 4π2 times this flux. The solar energy distribution curve may be approximated by a Planck blackbody curve at the effective temperature Teff (or bolometric temperature Tbol), defined as the temperature of a blackbody that would emit the same total energy as an emitting body, such as the Sun or a star. Then the Stefan-Boltzmann law implies L = 4π R 2 σ T4eff (e.g. J s-1 ) where σ is the Stefan-Boltzmann constant. Example: A star with surface temperature T = 1.2 x 104K • The SED peak for this star is at wavelength = 0.0029 / (1.2 x 104) m = (2.9/1.2) x 10-3-4 m = 2.4 x 10-7 m = 240 nm. PH507 Astrophysics Dr Dirk Froebrich -9- Physical Characteristics: statistical mechanics Stellar Atmospheres The spectral energy distribution of starlight is determined in a star’s atmosphere, the region from which radiation can freely escape. To understand stellar spectra, we first discuss a model stellar atmosphere and investigate the characteristics that determine the spectral features. The stellar photosphere, a thin, gaseous layer, shields the stellar interior from view. The photosphere is thin relative to the stellar radius, and so we regard it as a uniform shell of gas. The physical properties of this shell may be approximately specified by the average values of its pressure P, temperature T, and chemical composition µ (chemical abundances). We assume that the gas obeys the perfect-gas law: P =nkT where k is Boltzmann’s constant and n is the number of particles. This relationship is also known as Boyle’s Law. The Maxwell-Boltzmann Energy and Speed Distributions: Boltzmann Equation where Ni is the number of molecules at equilibrium temperature T, in a state i which has energy Ei and degeneracy gi, N is the total number of molecules in the system and k is the Boltzmann constant (denominator is called the partition function) PH507 Astrophysics Dr Dirk Froebrich - 10 - An important result that follows from it is that the (average) kinetic energy of a particle, or assemblage of particles, is given by the relationship; KE = 3 kT 2 Thus temperature is just a measure of the kinetic energy of a gas, or an assemblage of particles. This equation applies equally well to a star as a whole, as to a single particle, and later we will look at the comparison between a star’s kinetic and gravitational (potential) energies. The kinetic energy is also a measure of the speed that atoms or molecules are moving about at - the hotter they are, the faster they move. Thus, for a cloud of gas surrounding a hot star of temperature T = 15,000 K, which consists of hydrogen atoms (mass = 1.67 10-27 kg); the root mean square speed is given by: 3 1 2 kT = KE = mv 2 2 v= 3kT = 19 km s −1 ≅ 50,000 mph m The particle number density is related to both the mass density ρ(kg/m3) and the composition (or mean molecular weight) µ by the following definition of µ: 1 µ = mH n ρ where mH = 1.67 x 10-27 kg is the mass of a hydrogen atom. For a star of pure atomic hydrogen, µ = 1. If the hydrogen is completely ionised, µ = 1/2 because electrons and protons (hydrogen nuclei) are equal in number and electrons are far less massive than protons. In general: X = fraction of material by mass in form of hydrogen, Y = fraction of material by mass in form of helium, and Z = fraction of material by mass in form of heavier elements. Hence: X + Y + Z = 1. Now, in a fully ionised gas, PH507 Astrophysics Dr Dirk Froebrich - 11 - hydrogen gives 2 particles per mH (a proton and an electron), helium gives 3/4 particle per mH (a nucleus containing 2 protons and 2 neutrons = 4mH and two electrons) heavier elements ~1/2 particle per mH give (e.g. Carbon gives a nucleus containing 6 protons and 6 neutrons = 12mH and six electrons = 7/12, Oxygen gives a nucleus containing 8 protons and 8 neutrons = 16mH and eight electrons = 9/16, etc.) Thus per the number of particles cubic metre due to hydrogen = 2X ρ / mH, helium = 3Y ρ / 4mH, and heavier elements = Z ρ / 2mH. The total number of particles per cubic metre is then given by the sum of the above, i.e. n = (2X ρ / mH) + (3Y ρ / 4mH) + (Z ρ / 2mH). Rearranging, we obtain: n = (ρ / 4mH) (8X + 3Y + 2Z). Now, X + Y + Z = 1, and hence Z = 1 - X - Y, giving: n = (ρ / 4mH) (6X + Y + 2). Recalling that ρ = nmHµ, we can write: µ = 4 / (6X + Y + 2), which is a good approximation to µ except in the cool outer regions of stars. For solar composition, Xu=0.747, Yu=0.236 and Zu=0.017, resulting in µ~0.6, i.e. the mean mass of the particles in the Sun is a little over half the mass of a proton. PH507 Astrophysics Dr Dirk Froebrich - 12 - In general, stellar interior gases are ionised and µ= 1 3 1 2X + Y + Z 4 2 The mass fraction is the percentage by mass of one species relative to the total. Thus, for a pure hydrogen star (X=1.0, Y = 0.0, Z = 0.0), µ ~ 0.5, and for a white dwarf star (X = 0.0, Y = 1.0, Z = 0.0) µ ~ 1.33. Temperature Definitions The continuous spectrum, or continuum, from a star may be approximated by the Planck blackbody spectral-energy distribution. For a given star, the continuum defines a colour temperature by fitting the appropriate Planck curve. We can also define the temperature from Wien’s displacement law: λmaxT = 2.898 x 10-3 m . K, which states that the peak intensity of the Planck curve occurs at a wavelength λmax that varies inversely with the Planck temperature T. The value of λmax then defines a temperature. Also note here that the hotter a star is, the greater will be its luminous flux (in W/m2), in accordance with the Stefan-Boltzmann law: F = σT4 where σ= 5.67 x 10-8 W/m2 . K4. Then the relation L = 4πR2σT4eff defines the effective temperature of the photosphere. A word of caution: the effective temperature of a star is usually not identical to its excitation (Boltzmann eqn) or ionisation temperature (Saha eqn) because spectral-line formation redistributes radiation from the continuum. This effect is called line blanketing and becomes important when the numbers and strengths of spectral lines are large. PH507 Astrophysics Dr Dirk Froebrich - 13 - When spectral features are not numerous, we can detect the continuum between them and obtain a reasonably accurate value for the star’s effective surface temperature. The line blanketing alters the atmosphere’s blackbody character. Spectrophotometry: the Spectral Energy Distribution (SED) The goal of the observational astronomer is to make measurements of the EMR from celestial objects with as much detail, or the finest resolution, possible. There are of course different types of detail that we want to observe. These include angular detail, wavelength detail, and time detail. The perfect astronomical observing system would tell us the amount of radiation, as a function of wavelength, from the entire sky in arbitrarily small angular slices. We are always limited in angular and wavelength coverage, and limited in resolution in angle and wavelength. If we want good information about the wavelength distribution of EMR from an object (spectroscopy or spectrophotometry) we have to give up angular detail. If we want good angular resolution over a wide area of sky (imaging) we usually have to give up wavelength resolution or coverage. The ideal goal of spectrophotometry is to obtain the spectral energy distribution (SED) of celestial objects, or how the energy from the object PH507 Astrophysics Dr Dirk Froebrich - 14 - is distributed in wavelength. We want to measure the amount of energy received by an observer outside the Earth's atmosphere, per second, per unit area, per unit wavelength or frequency interval. Units of spectral flux (in cgs) look like: f λ = ergs s-1 cm-2 Å -1 if we measure per unit wavelength interval, or fν = ergs s-1 cm-2 Hz -1 (pronounced f nu if we measure per unit frequency interval). Classifying Stellar Spectra Observations A single stellar spectrum is produced when starlight is focused by a telescope onto a spectrometer or spectrograph, where it is dispersed (spread out) in wavelength and recorded photographically or electronically. If the star is bright, we may obtain a high-dispersion spectrum, that is, a few mÅ per millimetre on the spectrogram, because there is enough radiation to be spread broadly and thinly. At high dispersion, a wealth of detail appears in the spectrum, but the method is slow (only one stellar spectrum at a time) and limited to fairly bright stars. Dispersion is the key to unlocking the information in starlight. The Spectral-Line Sequence At first glance, the spectra of different stars seem to bear no relationship to one another. In 1863, however, Angelo Secchi found that he could crudely order the spectra and define different spectral types. Alternative ordering schemes appeared in the ensuing years, but the system developed at the Harvard Observatory by Annie J. Cannon and her colleagues was internationally adopted in 1910. This sequence, the Harvard spectral classification system, is still used today. (About 400,000 stars were classified by Cannon and published in various volumes of the Henry Draper Catalogue, 1910-1924, and its Extension, 1949. At first, the Harvard scheme was based upon the strengths of the hydrogen Balmer absorption lines in stellar spectra, and the spectral ordering was alphabetical (A through to P). Some letters were eventually dropped, and the ordering was rearranged to correspond to a sequence of PH507 Astrophysics Dr Dirk Froebrich - 15 - decreasing temperatures (see the effects of the Boltzmann and Saha equations): OBAFGKM. Stars nearer the beginning of the spectral sequence (closer to O) are sometimes called early-type stars, and those closer to the M end are referred to as late-type. Each spectral type is divided into ten parts from 0 (early) to 9 (late); for example, O3.5 . . . F8 F9 G0 G1 G2 . . . G9 K0 . . . . In this scheme, our Sun is spectral type G2. In 1922, the International Astronomical Union (IAU) adopted the Harvard system (with some modifications) as the international standard. Many mnemonics have been devised to help students retain the spectral sequence. A variation of the traditional one is “Oh, Be A Fine Girl, Kiss Me.” The next Figure shows exemplary stellar spectra arranged in order; note how the conspicuous spectral features strengthen and diminish in a characteristic way through the spectral types. PH507 Astrophysics Dr Dirk Froebrich - 16 - Comparison of spectra observed for seven different stars having a range of surface temperatures. The hottest stars, at the top, show lines of helium and multiply-ionised heavy elements. In the coolest stars, at the bottom, helium lines are not seen, but lines of neutral atoms and molecules are plentiful. At intermediate temperatures, hydrogen lines are strongest. The actual compositions of all seven stars are about the same. Additional Spectral Types: LTY: This is a continuation of the mass sequence for lower mass stars than M-dwarfs, which became necessary due to the discovery of these very low mass objects. L: temperatures 1300-2000K, some stellar, some sub-stellar, metalhydrates and alkali metal in spectra, >900 known T: cool brown dwarfs, 770-1000K, prominent methane lines in spectra, ~350 known Y: ultra cool brown dwarfs, <700K, 14 detected by WISE, WISE1828+2650 has T=298K PH507 Astrophysics Dr Dirk Froebrich - 17 - The Temperature Sequence The spectral sequence is a temperature sequence, but we must carefully qualify this statement. There are many different kinds of temperatures and many ways to deter-mine them. (I.e. it might be better to talk of a mass sequence, at least for the luminosity class V objects – the main sequence stars.) Theoretically, the temperature should correlate with spectral type and so with the star’s colour. From the spectra of intermediate-type stars (A to K), we find that the (continuum) colour temperature does so, but difficulties occur at both ends of the sequence. For O and B stars, the continuum peaks in the far ultraviolet, where it is undetectable by ground-based observations. Through satellite observations in the far ultraviolet, we are beginning to understand the ultraviolet spectra of O and B stars. For the cool M stars, not only does the Planck curve peak in the infrared, but numerous molecular bands also blanket the spectra of these lowtemperature stars. PH507 Astrophysics Dr Dirk Froebrich - 18 - PH507 Astrophysics Dr Dirk Froebrich - 19 - When the strengths of various spectral features are plotted against excitation-ionisation (or Boltzmann-Saha) temperature; the spectral sequence does correlate with this temperature as seen below; In practice, we measure a star’s colour index, CI = B - V, to determine the effective stellar temperature. If the stellar continuum is Planck-like and contains no spectral lines, this procedure clearly gives a unique temperature, but observational uncertainties and physical effects do lead to problems: (a) for the very hot O and B stars, CI varies slowly with Teff and small uncertainties in its value lead to very large uncertainties in T; (b) for the very cool M stars, CI is large and positive, but these faint stars have not been adequately observed and so CI is not well determined for them; (c) any instrumental deficiencies, calibration errors, or unknown blanketing in the B or V bands affect the value of CI - and thus the deduced T. Hence, it is best to define the CI versus T relation observationally. PH507 Astrophysics Dr Dirk Froebrich - 20 - SPECTROSCOPY • We have discussed stellar spectra and classification on an empirical basis: Spectral sequence O B A F G K M Temperature ~40,000 K ----> 2500 K Classification based on relative line strengths of He, H, Ca, metal, molecular lines. • We will now look a little deeper at stellar spectra and what they tell us about stellar atmospheres. Radiative Transfer Equation Imagine a beam of radiation of intensity Iλ passing through a layer of gas: Power passing into volume Area dA Eλ = Iλ dω dA dλ Power passing out of volume E λ + dE λ where Iλ = intensity into solid angle element dω path length ds NB in all these equations subscripts λ can be replaced by ν In the volume of gas there is: ABSORPTION - Power is reduced by amount dEλ = - κλ ρ Eλ ds = - κλ ρ Iλ dω dA dλ ds where κλ is the ABSORPTION COEFFICIENT or OPACITY = the cross-section for absorption of radiation of wavelength λ (frequency ν) per unit mass of gas. Units of κλare m2 kg-1 The quantity κλ ρ is the fraction of power in a beam of radiation of wavelength λ absorbed by unit depth of gas. It has units of m-1. (NB in many texts κλ ρ is simply given the symbol κλ in the equations given here - beware!) PH507 Astrophysics Dr Dirk Froebrich - 21 - EMISSION - Power is increased by amount dEλ = jλ ρ dω dA dλ ds (1) where jλ = EMISSION COEFFICIENT = amount of energy emitted per second per unit mass per unit wavelength into unit solid angle. Units of jλ (jν) are Wkg-1µm-1sr-1 (Wkg-1Hz-1sr-1) or ms-3sr-1 (NB power production per unit volume per unit wavelength into unit solid angle is ελ=jλ ρ. More confusion is possible here, since ε is also the symbol used for total power output of a gas, units are Wkg-1, Beware!) So total change in power is dEλ = dIλ dω dA dλ = - κλ ρ Iλ dω dA dλ ds + jλ ρ dω dA dλ ds which reduces to dIλ = - κλ ρ Iλ ds + jλ ρ ds dIλ ds = - κ λ ρ Iλ + jλ ρ (2) (3) This is a form of the radiative transfer equation in the plane parallel case. Optical depth • Take a volume of gas which only absorbs radiation (jλ = 0) at λ : dIλ = - κλ ρ Iλ ds For a depth of gas s, the fractional change in intensity is given by I (s) λ dI ∫ I I (0) λ s λ λ = ∫0 - κ λ ρ ds s I (s) ln ( Integrating ==> λ I (0) ) = - λ s - ==> I (s) = I (0) e λ λ ∫ κλ ρ ds 0 ∫0 κ λ ρ ds PH507 Astrophysics Dr Dirk Froebrich - 22 - We define Optical Depth τ s τ ∫κ = λ 0 λ ρ ds (4) -τ λ I (s) = I (0) e So λ (5) λ • Intensity is reduced to 1/e (=1/2.718 = 0.37 ) of its original value if optical depth τ= 1. • Optical depth is not a physical depth. A large optical depth can occur in a short physical distance if the absorption coefficient κ is large, or a large physical distance if κ is small. Full Radiative Transfer Equation again dIλ = - κ λ ρ Iλ + jλ ρ ds divide by ρ κλ: dI j λ κ ρ ds = -I + λ λ λ κ λ dI λ dτ = -I + S λ λ (6) As ds --> 0, κλ is constant over ds: This is the RADIATIVE TRANSFER EQUATION in the plane parallel case. Define: λ j S = λ where λ κ or j = κ S λ λ λ λ Sλ is the SOURCE FUNCTION. Radiative transfer in a blackbody • Remember definition of a blackbody as a perfect absorber and emitter of radiation. Matter and radiation are in THERMODYNAMIC EQUILIBRIUM, i.e. gross properties do not change with time. Therefore a beam of radiation in a blackbody is constant: PH507 Astrophysics dIλ ds Dr Dirk Froebrich - 23 - = 0 = - κ λ ρ Iλ + jλ ρ from definition of source function, jλ = κλ Sλ ==> 0 = κλ (Iλ - Sλ), i.e. Iλ = Sλ. but for a blackbody Iλ = Bλ the PLANCK FUNCTION 2 B = λ 2hc λ5 3 1 B = hc/λkT (e - 1) ν 2hν 2 c 1 hν/kT (e - 1) Summary: in complete thermodynamic equilibrium the source function equals the Planck function, (7) i.e. jλ = κλ Bλ (Kirchoff's Law). • In studies of stellar atmospheres we make the assumption of LOCAL THERMODYNAMIC EQUILIBRIUM (LTE), i.e. thermodynamic equilibrium for each particular layer of a star. • Note that if incoming radiation at a particular wavelength (e.g. in a spectral line) enters a blackbody gas it is absorbed, but emission is distributed over all wavelengths according to the Planck function. All information about the original energy distribution of the radiation is lost. This is what happens in interior layers of a star where the density is high and photons of any wavelength are absorbed in a very short distance. Such a gas is said to be optically thick (see below). Emission and Absorption lines • κλ the absorption coefficient describes the efficiency of absorption of material in the volume of gas. In a low density gas, photons can generally pass through without interaction with atoms unless they have an energy corresponding to a particular transition (electron energy level transition, or vibrational/rotational state transition in molecules). At this particular energy/frequency/wavelength the absorption coefficient κλ is large. • Let's imagine the volume of gas shown earlier with both absorption and emission: PH507 Astrophysics Dr Dirk Froebrich - 24 - Iλ I λ(0) path length s dI λ = S - I λ dτ λ λ Multiply both sides by eτλ and re-arrange dI ==> τ τ τ eλ + I eλ = S eλ λ λ dτ λ λ d ==> τ τ (I e λ) = S e λ dτ λ λ λ integrate over whole volume, i.e. from 0 to s, or 0 to τλ τ τ I eλ λ ==> τ λ = 0 τ S eλ λ λ 0 assuming Sλ = constant along path ==> Iλ eτλ - Iλ(0) = Sλ eτλ - Sλ ==> Iλ Iλ(0) e-τλ + radiation left over from light entering box. = Sλ (1 - e-τλ ) light from radiation emitted in the box. (8) τλ >> 1: OPTICALLY THICK CASE If τλ >> 1, then e-τλ --> 0, and eqn (8) becomes Iλ = Sλ (9) In LTE Sλ = Bλ, the Planck function. So for an optically thick gas, the emergent spectrum is the Planck function, independent of composition or input intensity distribution. True for stellar photosphere (the visible "surface" of a star). • Case 1 PH507 Astrophysics Dr Dirk Froebrich τλ << 1 OPTICALLY THIN CASE If τλ << 1, then e-τλ ≈ 1 - τλ (first two terms of Taylor series expansion) eqn (8) becomes Iλ = Iλ(0) (1 - τλ) + Sλ (1 - 1 + τλ) ==>Iλ = Iλ(0) + τλ ( Sλ - Iλ(0) ) - 25 - • Case 2 (10) • If Iλ(0) = 0 : no radiation entering the box (from direction of interest): From eqn (8) Iλ = τλ Sλ (= τλ Bλ in LTE) Since τλ = ∫ κλ , then Iλ = κλ ρ s Sλ If κλ is large (true at wavelength of spectral lines) then Iλ is large, we see EMISSION LINES. This happens for example in gaseous nebulae or the solar corona when the sun is eclipsed. • If Iλ(0) ≠ 0 , let's examine eqn (8) Iλ = Iλ(0) + τλ ( Sλ - Iλ(0) ) If Sλ > Iλ(0) then right hand term is positive when τλ is large (i.e. κλ is large) we see higher intensity than Iλ(0) ==> EMISSION LINES ON BACKGROUND INTENSITY. If Sλ < Iλ(0) then right hand term is negative when τλ is large (ie κλ is large) we see lower intensity than Iλ(0) ==> ABSORPTION LINES ON BACKGROUND INTENSITY. For stars we see absorption lines. This means Iλ(0) > Sλ, i.e. (intensity from deeper layers) > (source function for the top layers Assuming LTE (Sλ = Bλ) the source function increases as temperature increases: Iλ(0) = Bλ(Tdeep layer) > Sλ = Bλ(Touter layer). Therefore temperature must be increasing as we go into the star for absorption lines to be observed. • To summarise: 4 possibilities - We see CONTINUUM RADIATION for an optically thick gas (= PLANCK FUNCTION assuming LTE). - We see EMISSION LINES for an optically thin gas. - We see ABSORPTION LINES + CONTINUUM for an optically thick gas overlaid by optically thin gas with temperature decreasing outwards. - We see EMISSION LINES + CONTINUUM for an optically thick gas overlaid by an optically thin gas with temperature increasing outwards. PH507 Astrophysics Dr Dirk Froebrich - 26 - Atomic Spectra - Absorption & Emission line series and continua • Bohr theory (last year's physics unit) adequately describes electron energy levels in Hydrogen. Quantum mechanics is required for more massive atoms to describe the dynamics of electrons. However, we are interested here only in the energy levels of electron states rather than a detailed model or description of atomic structure. We can therefore use ENERGY LEVEL DIAGRAMS without worrying too much about the theory behind them. • There are 3 basic photon absorption mechanisms related to electrons. Using Hydrogen as the example, the electron energy levels are given by the principal quantum number n, as: E(n) = - me e4 / 32 π2 ε02 n2 ћ2 from Bohr Theory The lowest energy level of H (n = 1) is about -13.6 eV. The next energy level (n = 2) is -3.4 eV. The third (n = 3) is -1.51 eV Opacity. We first introduced the concept of opacity when deriving the equation of radiative transport. Opacity is the resistance of material to the flow of radiation, which in most stellar interiors is determined by all the processes which scatter and absorb photons. We will now look at each of these processes in turn, of which there are four: • • • • bound-bound absorption bound-free absorption free-free absorption scattering The first three are known as true absorption processes because they involve the disappearance of a photon, whereas the fourth process only alters the direction of a photon. All four processes are described below and are shown pictorially in figure 1. PH507 Astrophysics Dr Dirk Froebrich - 27 - Figure 1 : Schematic energy level diagram showing the four microscopic processes which contribute to opacity in stellar interiors. bound-bound absorption Bound-bound absorptions occur when an electron is moved from one orbit in an atom or ion into another orbit of higher energy due to the absorption of a photon. If the energy of the two orbits is E1 and E2, a photon of frequency νbb will produce a transition if E2 - E1 = hνbb. Bound-bound processes are responsible for the spectral lines visible in stellar spectra, which are formed in the atmospheres of stars. In stellar interiors, however, bound-bound processes are not of great importance as most of the atoms are highly ionised and only a small fraction contain electrons in bound orbits. In addition, most of the photons in stellar interiors are so energetic that they are more likely to cause bound-free absorptions, as described below. PH507 Astrophysics Dr Dirk Froebrich - 28 - bound-free absorption Bound-free absorptions involve the ejection of an electron from a bound orbit around an atom or ion into a free hyperbolic orbit due to the absorption of a photon. A photon of frequency νbf will convert a bound electron of energy E1 into a free electron of energy E3 if E3 - E1 = hνbf. Provided the photon has sufficient energy to remove the electron from the atom or ion, any value of energy can lead to a bound-free process. Boundfree processes hence lead to continuous absorption in stellar atmospheres. In stellar interiors, however, the importance of bound-free processes is reduced due to the rarity of bound electrons. free-free absorption Free-free absorption occurs when a free electron of energy E3 absorbs a photon of frequency νff and moves to a state with energy E4, where E4 - E3 = hνff. There is no restriction on the energy of a photon which can induce a freefree transition and hence free-free absorption is a continuous absorption process which operates in both stellar atmospheres and stellar interiors. Note that, in both free-free and bound-free absorption, low energy photons are more likely to be absorbed than high energy photons. scattering In addition to the above absorption processes, it is also possible for a photon to be scattered by an electron or an atom. One can think of scattering as a collision between two particles which bounce of one another. If the energy of the photon satisfies hν << mc2, where m is the mass of the particle doing the scattering, the particle is scarcely moved by the collision. In this case the photon can be imagined to be bounced off a stationary particle. Although this process does not lead to the true absorption of radiation, it does slow the rate at which energy escapes from a star because it continually changes the direction of the photons. PH507 Astrophysics Dr Dirk Froebrich - 29 - Bound-Bound Transitions • BOUND - BOUND transitions give rise to spectral lines. • ABSORPTION LINE if a photon is absorbed, causing increase in energy of an electron. Energy of absorbed photon: hν = E(nu) - E(nl) (1) where E(nu) and E(nl) are energies of upper and lower energy levels respectively. This is RADIATIVE EXCITATION. • Note energy can also be absorbed through collisions of a free particle (COLLISIONAL EXCITATION) - no absorption line is seen in this case. • Atom remains in excited state until SPONTANEOUS EMISSION (photon is emitted typically after ~10-8s) or INDUCED EMISSION (Photon emitted at same energy and coherently with incoming photon - as in lasers – stimulated emission). Both produce EMISSION LINES. • Narrow lines are seen since transitions can only occur if photon has energy (frequency/wavelength) corresponding to difference in energy levels • Energy level diagram shows electron energy level changes for absorption of a photon. Lowest energy level set to zero energy. 1eV = 1.6 x 10-19 J. 13.6 eV n=∝ n=4 n=3 12.73 eV 12.07 eV n=2 10.19 eV n=1 0 eV Lyman Series Balmer Paschen Series Series • Series of lines seen -LYMAN-SERIES transitions to/from n=1 lines seen in UV -BALMER-SERIES -"n=2 -“visual -PASCHEN-SERIES -"n=3 -“. infrared ... PH507 Astrophysics Dr Dirk Froebrich 30 Bound-free transitions • If photon has energy greater than that required to move an electron in an atom from its current energy level to level n=∞, the electron will be released, ionizing the atom. • Ionization potential for Hydrogen is X = 13.6 eV. • Energy of absorbed photon is hν = (X - E(nl)) + mev 2 /2 (48) 1/2 m ev 2 13.6 eV n=∝ n=4 n=3 12.73 eV 12.07 eV n=2 10.19 eV n=1 0 eV • Since one of the states (free electron) can have any energy, the transition can have any energy and the photon any frequency (above a certain value determined by X and E(nl)). Thus BOUND-FREE transitions give an ABSORPTION CONTINUUM. • RE-COMBINATION is a FREE-BOUND transition and results in an EMISSION CONTINUUM. • The spectrum produced by absorption from a single energy level will therefore appear as a series of lines of increasing energy continuum ∝ δ γ ν β α λ (Increasing frequency, decreasing wavelength) up to a limit defined by XE(nl), with an absorption continuum shortward of this limit. The characteristic of a bound-free transition in a spectrum is an edge: no absorption below some energy, then a sharp onset in the absorption above that critical energy. As we’ll see, the absorption decreases above the critical energy. PH507 Astrophysics Dr Dirk Froebrich 31 • For nl=1 the Lyman series (Lyman-α 121.57nm, Lyman-β 102.57nm, etc.) is observed together with the Lyman continuum shortward of λ=91.2 nm. (Since interstellar space is populated by very low density and low temperature hydrogen (i.e. with n=1), photons with λ<91.2nm are easily absorbed so it is opaque in the near-UV). For nl=2 the Balmer series (Hα 656.28nm, Hβ 486.13nm, etc.) is observed together with the Balmer continuum shortward of λ=364.7 nm. Free-free transitions • Absorption of a photon by a free electron in the vicinity of an ion. Electron changes from free energy state with velocity v1 to one with velocity v2 i.e. hν = 1/2 me v22 - 1/2 me v12 The term means that the inverse process “braking radiation” occurs when an electron is accelerated by passage near an ion, and hence radiates. Bremsstrahlung and free-free absorption are basic radiative processes that show up in many contexts. When X-rays and gamma-rays are considered, we’ll talk about the more general process of Compton scattering (heating the electrons) and inverse Compton cooling. Cyclotron and Synchrotron Radiation: When magnetic fields are present, charges can interact with them and radiate or absorb radiation. For slowly moving particles this happens at a single frequency, the cyclotron frequency. For relativistically moving particles, the emission or absorption occurs over a large range of frequencies, and is called in this case synchrotron radiation. Determination of κλ • The actual spectrum of a star depends on the physical conditions (notably temperature) and composition of the stellar atmosphere. The intensity is produced at a physical level in the star where τλ ~ 2/3. In order to determine the total spectrum, the value of κλ needs to be determined at all wavelengths. The overall κλ is the sum of the contributions from each atomic/molecular species in the atmosphere. Each component of κλ depends on the number of atoms/molecules with a given energy state capable of absorbing radiation at that frequency and the absorption efficiency. We deal with the energy state populations first: PH507 Astrophysics Dr Dirk Froebrich 32 Boltzmann's equation (Excitation equilibrium) • Boltzmann's equation describes the population distribution of energy states for a particular atom in a gas. The ratio of number of atoms unit volume (per m3) in energy state B to energy state A: NB NA gB (EA - EB)/kT e gA = (50) where gA and gB are STATISTICAL WEIGHTS (number of different quantum states of the same energy), k = Boltzmann const and T = temperature of gas. EB > EA so exponential power is negative. • The probability of finding an atom in an excited state decreases exponentially with the energy of the excited state, but increases with increasing temperature. Saha Equation (Ionization Equilibrium) • The Boltzmann eqn does not describe all the possible atomic states. Excitation may cause electrons to be lost completely. There are therefore a number of different ionization states for a given atom, each of which has one or more energy states. • The ratio of the number of atoms of ionization state i+1 to those of ionization state i (i=I is neutral, i=II is singly ionized, etc) is given by 3/2 Ni+1 Ni = Ui+1 2 Ui Ne 2π me k T h 2 -Χi /kT e where Ne is the electron density (number of electrons per m3), Xi is the ionization potential of the ith ionization state, Ui+1 and Ui are PARTITION FUNCTIONS obtained from the statistical weights: ∞ Ui = gi 1 + ∑ -Ei n /kT gi n e n =2 • The higher the Ionization Potential, Xi, the lower the fraction of atoms in the upper ionization state. The higher the Temperature, T, the higher the fraction of atoms in the upper ionization state, (Collisional excitation is more likely to ionize atom), The higher the electron density, the lower the fraction of atoms in the upper ionization state (due to re-combination). PH507 Astrophysics Dr Dirk Froebrich 33 • The Boltzmann and Saha Equations give the fraction of atoms in a given ionization state and energy level allowing (when combined with absorption/emission probabilities) κλ and hence the line strengths to be related to abundances. Example - Abundances in the Sun • In line forming regions in the Sun: Gas Hydrogen Calcium T ~ 6000 K, Ne ~ 7x1019 m-3. ΧI ΧII UII/UI UIII/UII 13.6 eV 2 6.1 eV 11.9 eV ~2 ~0.5 g1 2 1 g2 2 6 Hydrogen: From Saha Equ. for Hydrogen, the ratio of ionized to un-ionized H atoms NII/NI ≈ 6x10-5, i.e. most of Hydrogen is un-ionized. From Boltzmann equation, ratio of number of atoms with electrons in level n=2 to those in level n=1 (E1-E2 = -10.19 eV) is N2/N1 ≈ 3x10-9, i.e almost all H atoms are in the ground state. The H Balmer lines which originate from level n=2 are strong only because the H abundance is so high. Calcium: From Saha Equation for Calcium, NII/NI ≈ 600 and NIII/NII ≈ 2x10-3 i.e. most of Calcium is in the singly ionized state. From Boltzmann equation, ratio of number of atoms with electrons in energy states which contribute to the H and K lines to those in the ground state (E1-E2 = -3.15, -3.13 eV) is (NB/NA)II ≈ 10-2, i.e most Ca atoms are in the ground state. The H and K lines of Calcium are therefore strong because most Ca atoms in the Sun are in an energy state capable of producing the lines. • For stars cooler than the Sun more H is in the ground state so Balmer lines will be weaker, for stars hotter than the Sun more H is in n=2 state so Balmer lines will be stronger. (T ~ 85000 K needed for N2/N1 =1). But at this temperature NII/NI = 105 so little remains un-ionized. PH507 Astrophysics Dr Dirk Froebrich 34 • Balmer line strength depends on excitation (function of T) and ionization (function of T and Ne). Balance of effects occurs at T ~ 10,000 K so Balmer lines are strongest in A0 stars. • A similar effect occurs for other species but at different temperatures. Transition probabilities • Once we know the population of all energy states for a given gaseous species we need to know the transition probabilities for each energy state change before the absorption coefficient can be determined. • The transition probabilities must be calculated from atomic theory or determined by experiment - much time has been invested in this major problem in astrophysics. • The EINSTEIN TRANSITION PROBABILITY (inverse of lifetime): for spontaneous emission, A21 ∝ ν2 for stimulated emission B21 ∝ ν-1 for absorption A12 ∝ ν-1 Total κλ • We can now calculate κλ for a given gaseous species. For Hydrogen (removing spectral line opacities for clarity): Lyman continuum absorption κ falls off with decreasing λ λ due to ν -1 dependence Log κλ T~25000K (B star) Balmer continuum absorption Paschen continuum absorption T~5000K (G star) 30 100 300 1000 λ(nm) • Similar diagrams exist for other species. The total κλ will be the sum for all species in the star. • The region of a star for which optical depth τ~2/3 determines where observed radiation originates. So if κλ is large, then τλ= 2/3 at a high level in the atmosphere and if κλ is low, τλ= 2/3 deep in the atmosphere. PH507 Astrophysics Dr Dirk Froebrich 35 Solar photospheric opacity • The solar atmosphere is dominated by hydrogen. The visible surface, the photosphere, has a temperature ~5800 K. However, as can be seen from the diagram above, κλ for hydrogen at low temperatures is low in the visible region (λ~400-700nm). This is because the continuum absorption in the visible is due to Paschen absorption (electrons originating in level n=3) and most hydrogen is in ground state or n=2 level. We would therefore expect the continuum to come from much deeper in the sun where temperatures are higher. So what causes the high solar photospheric opacity? The solar opacity comes from the H- ion. The ionization potential for H- --> H + eis 0.75 eV (λ=1650nm). From Boltzmann eqn, for H: N3/N1 = 6 x 10-10 But from Saha eqn N(H)/N(H-) ≈ 3 x 107 Therefore N(H-)/N3 ≈ 500. PH507 Astrophysics Log κλ Dr Dirk Froebrich 36 T~25000K (B star) H - bound-free H - free-free T~5000K (G star) 30 100 300 1000 λ(nm) i.e. number of H- ions is greater than number of H atoms in level n=3, so absorption of photons to dissociate H- to H dominates the continuum absorption in the optical. Limb darkening • The Sun is less bright near the limb than at the centre of the disk. • The continuum spectrum of the entire solar disk defines a StefanBoltzmann effective temperature of 5800 K for the photosphere, but how does the temperature vary in the photosphere? A clue is evident in a white-light photograph of the Sun. • We see that the brightness of the solar disk decreases from the centre to the limb - this effect is termed limb darkening. PH507 Astrophysics Dr Dirk Froebrich 37 Limb darkening arises because we see deeper, hotter gas layers when we look directly at the centre of the disk and higher, cooler layers when we look near the limb. Assume that we can see only a fixed distance d through the solar atmosphere. The limb appears darkened as the temperature decreases from the lower to the upper photosphere because, according to the Stefan-Boltzmann law (Section 8-6), a cool gas radiates less energy per unit area than does a hot gas. The top of the photosphere, or bottom of the chromosphere, is defined as height = 0 km. Outward through the photosphere, the temperature drops rapidly then again starts to rise at about 500 km into the chromosphere, reaching very high temperatures in the corona. PH507 Astrophysics Dr Dirk Froebrich 38 Formation of solar absorption lines. Photons with energies well away from any atomic transition can escape from relatively deep in the photosphere, but those with energies close to a transition are more likely to be reabsorbed before escaping, so the ones we see on Earth tend to come from higher, cooler levels in the solar atmosphere. The inset shows a close-up tracing of two of the thousands of solar absorption lines, those produced by calcium at about 395 nm. PH507 Astrophysics Dr Dirk Froebrich 39 At this point, you may have discerned an apparent paradox: how can the solar limb appear darkened when the temperature rises rapidly through the chromosphere? Answering this question requires an understanding of the concepts of opacity and optical depth. Simply put, the chromosphere is almost optically transparent relative to the photosphere. Hence, the Sun appears to end sharply at its photospheric surface - within the outer 300 km of its 700,000 km radius. Our line of sight penetrates the solar atmosphere only to the depth from which radiation can escape unhindered (where the optical depth is small). Interior to this point, solar radiation is constantly absorbed and re-emitted (and so scattered) by atoms and ions. Spectral line formation • Lines form higher in atmosphere than continuum. For optical lines this corresponds to lower temperature than continuum and therefore lower intensity (absorption lines) (see p18 where Sλ < Iλ). κ small τ~2/3 low in atmosphere 6500 T (K) κ high τ~2/3 high in atmosphere 4500 Fλ λ 0 200 400 km Height above photosphere Spectral line strength Spectral lines are never perfectly monochromatic. Quantum mechanical considerations govern minimum line width, and many other processes cause line broadening : Shape of absorption line — line profile. Natural broadening — consequence of uncertainty principle. Doppler broadening — consequence of velocity distribution. Pressure broadening — perturbation of energy levels by ions. PH507 Astrophysics Dr Dirk Froebrich 40 • For abundance calculations we want to know the total line strength. Total line strength is characterised by EQUIVALENT WIDTH. Equivalent width: measure strength of lines. Rectangle with same area as line, i.e. same amount of absorption. EW is width in °A across rectangle Need EW to determine number of absorbing atoms Stellar composition • Derived from spectral line strengths in stellar atmospheres. In the solar neighbourhood, the composition of stellar atmospheres is: Element H He C,N,O,Ne,Na,Mg,Al,Si,Ca,Fe, others % mass 70 28 ~2. Spectral line structure • NATURAL WIDTH: Due to uncertainty principle, ∆E=h/∆t, applied to lifetime of excited state. For "normal" lines the atom is excited (by a photon or collision) to an excited state which has a short lifetime ∆t ~ 10-8 s. The upper energy level therefore has uncertain energy ∆E and the resultant spectral line (absorption or emission) has an uncertain energy (wavelength). The line has a Lorentz profile, ∆λ ~ 10-5 nm for visible light. • COLLISIONAL/PRESSURE BROADENING: Outer energy levels of atoms affected by presence of neighbouring charged particles (ions and electrons). Random effects lead to line broadening since the energy of upper energy level changes relative to the unexcited state energy level. This is the basis of the Luminosity classification for A,B stars. Gaussian profile. ∆λ ~ 0.02 - 2 nm. • DOPPLER BROADENING: Due to motions in gas producing the line. Doppler shift occurs for each each photon emitted (or absorbed) since the gas producing the line is moving relative to the observer (or gas producing the photon). Thermal Doppler broadening due to motions of individual atoms in the gas. ~0.01 -0.02 nm for Balmer lines in the Sun. Gaussian profile. Bulk motions of gas in convection cells. Gaussian profile. PH507 Astrophysics Dr Dirk Froebrich 41 • ROTATION: If there is no limb darkening, then lines have hemispherical profile due to combination of radiation from surface elements with different radial velocities. Effect depends on rotation rate, size of star and angle of polar tilt. In general, v*sin(i) is derived from the profile. _ V (km s -1) 200 Receding +V A F λ C B A C 100 B Approaching -V λ λo 0 O B A F G K • ATMOSPHERIC OUTFLOW: Many different types. Star with expanding gas shell (result of outburst) gives P-CYGNI PROFILE. Continuum (+ absorption lines) from star, emission or absorption lines from shell: F Expanding gas shell D C Star D B λ D A λo λ Observer B C A C B Radiation from star, A, passes through cooler cloud giving absorption line due to shell material which is blue shifted relative to star. Elsewhere, emission lines are seen. PH507 Astrophysics Dr Dirk Froebrich C Fλ Rotating gas shell 42 E Star B A λo D λ Observer C B A D E Be STARS: Very rapid rotators with material lost from the equator: Radiation from star, A, passes through cooler cloud giving absorption line. Overall line structure is hemispherical rotation line (B,D). Emission lines seen due to shell material (C,E). Forbidden lines • Only certain transitions are generally seen for two reasons: 1) Outer energy levels are far from the nucleus so in dense gases, levels are distorted or destroyed by interactions. 2) Selection rules for change of quantum numbers restrict possible transitions. • In fact forbidden transitions are not actually forbidden. However, the probability of a forbidden transition is very low, so an allowed transition will generally occur. The lifetimes in an excited state for which there are no allowed downward transitions are ~10-3 - 109 seconds (i.e. very low transition probability). These are called METASTABLE STATES. • De-excitation from a metastable state can be by: 1) Collisional excitation, or absorption of another photon to higher energy state allowing another downward transition to the equilibrium state, 2) FORBIDDEN TRANSITION producing a FORBIDDEN LINE. Usually denoted with [], e.g. [OII 731.99]. • Forbidden lines are usually much fainter than those from allowed transitions due to low probability. • In interstellar nebulae excited by UV from nearby hot stars, some elements' excited states have no allowed downward transitions to the ground state. In the absence of frequent collisions (due to low density) or high photon flux, a forbidden transition is the only way to the ground state. • These lines were not understood for a long while. A new element Nebulium was invented to account for them. • “Forbidden lines are allowed in 99.999% of the Universe!” PH507 Astrophysics Dr Dirk Froebrich 43 Radiation Mechanisms 1. 21 cm Hydrogen gas is observed in a variety of states: in ionized, neutral atomic, and molecular forms. The ionized hydrogen emits light in the visible band as the electrons recombine with the protons and the neutral atomic and molecular hydrogen emits light in the radio band of the electromagnetic spectrum. Most of the hydrogen in space (far from hot O and B-type stars) is in the ground state. The electron moving around the proton can have a spin in the same direction as the proton's spin (i.e., parallel) or spin in the direct opposite direction as the proton's spin (i.e., anti-parallel). The energy state of an electron spinning anti-parallel is slightly lower than the energy state of a parallel-spin electron. Remember that the atom always wants to be in the lowest energy state possible, so the electron will eventually flip to the anti-parallel spin direction if it was somehow knocked to the parallel spin direction. The energy difference is very small, so a hydrogen atom can wait on average a few million years before it undergoes this transition. The two levels of the hydrogen 1s ground state, slightly split by the interaction between the electron spin and the nuclear spin. The splitting is known as hyperfine structure. Even though this is a RARE transition, the large amount of hydrogen gas means that enough hydrogen atoms are emitting the 21-cm line radiation at any one given time to be easily detected with radio telescopes. Our galaxy, the Milky Way, has about 3 billion solar masses of H I gas with about 70% of it further out in the Galaxy than the Sun. Most of the H I gas is in disk component of our galaxy and is located within 720 light years from the midplane of the disk. What's very nice is that 21-cm line radiation is not blocked by dust! The 21-cm line radiation provides the best way to map the structure of the Galaxy. http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/h21.html 2. Thermal free-free or Bremsstrahlung emission Another form of thermal emission comes from gas which has been ionized. Atoms in the gas become ionized when their electrons become stripped or PH507 Astrophysics Dr Dirk Froebrich 44 dislodged. This results in charged particles moving around in an ionized gas or "plasma", which is a fourth state of matter, after solid, liquid, and gas. As this happens, the electrons are accelerated by the charged particles, and the gas cloud emits radiation continuously. This type of radiation is called "free-free" emission or "bremsstrahlung". 3. Synchrotron radiation Non-thermal emission does not have the characteristic signature curve of blackbody radiation. In fact, it is quite the opposite, with emission increasing at longer wavelengths.The most common form of non-thermal emission found in astrophysics is called synchrotron emission. Basically, synchrotron emission arises by the acceleration of charged particles within a magnetic field. Most commonly, the charged particles are electrons. Compared to protons, electrons have relatively little mass and are easier to accelerate and can therefore more easily respond to magnetic fields. Click to animate! Reset As the energetic electrons encounter a magnetic field, they spiral around it rather than move across it. Since the spiral is continuously changing the direction of the electron, it is in effect accelerating, and emitting radiation. The frequency of the emission is directly related to how fast the electron is traveling. This can be related to the initial velocity of the electron, or it can be due to the strength of the magnetic field. A stronger field creates a tighter spiral and therefore greater acceleration. For this emission to be strong enough to have any astronomical value, the electrons must be traveling at nearly the speed of light when they encounter a magnetic field; these are known as "relativistic" electrons. (Lower-speed interactions do happen, and are called cyclotron emission, but they are of considerably lower power, and are virtually non-detectable astronomically). As the electron travels around the magnetic field, it gives up energy as it emits photons. The longer it is in the magnetic field, the more energy it loses. As a result, the electron makes a wider spiral around the magnetic field, and emits EM radiation at a longer wavelength. To maintain synchrotron radiation, a continual supply of relativistic electrons is necessary. Typically, these are supplied by very powerful energy sources such as supernova remnants, quasars, or other forms of active galactic nuclei (AGN). It is important to note that, unlike thermal emission, synchrotron emission is polarized. As the emitting electron is viewed side-on in its spiral motion, PH507 Astrophysics Dr Dirk Froebrich 45 it appears to move back-and-forth in straight lines. Its synchrotron emission has its waves aligned in more or less the same plane. At visible wavelengths this phenomenon can be viewed with polarized lenses (as in certain sunglasses, and in modern 3-D movie systems). Synchrotron radiation is electromagnetic radiation, similar to cyclotron radiation, but generated by the acceleration of ultrarelativistic (i.e., moving near the speed of light) electrons through magnetic fields. This may be achieved artificially by storage rings in a synchrotron, or naturally by fast moving electrons moving through magnetic fields in space. The radiation typically includes infrared, optical, ultraviolet, x-rays. Synchrotron radiation is also generated by astronomical structures and motions, typically where relativistic electrons spiral (and hence change velocity) through magnetic fields. Two of its characteristics include (1) Non-thermal radiation (2) Polarization. 4. inverse Compton radiation Inverse Compton scattering is important in astrophysics. In X-ray astronomy, the accretion disk surrounding a black hole is believed to produce a thermal spectrum. The lower energy photons produced from this spectrum are scattered to higher energies by relativistic electrons in the surrounding corona. This is believed to cause the power law component in the X-ray spectra (0.2-10 keV) of accreting black holes. The effect is also observed when photons from the Cosmic microwave background move through the hot gas surrounding a galaxy cluster. The CMB photons are scattered to higher energies by the electrons in this gas, resulting in the Sunyaev-Zel'dovich effect. The Inverse Compton process boosts up synchrotron photons by means of scattering against the high energy electrons. Since that the electrons that scatter against the synchrotron photons, belong to the same seed of the electrons that have produced the synchrotron photons, this process is also called ``Self Synchrotron Compton'' or SSC 5. Masers Another form of non-thermal emission comes from masers. A maser, which stands for "microwave amplification by stimulated emission of radiation", is similar to a laser (which amplifies radiation at or near visible wavelengths). Masers are usually associated with molecules, and in space masers occur naturally in molecular clouds and in the envelopes of old stars. Maser action amplifies otherwise faint emission lines at a specific PH507 Astrophysics Dr Dirk Froebrich 46 frequency. In some cases the luminosity from a given source in a single maser line can equal the entire energy output of the Sun from its whole spectrum. Masers require that a group of molecules be pumped to an energized state (labeled E2 in the diagram at right), like compressed springs ready to uncoil. When the energized molecules are exposed to a small amount of radiation at just the right frequency, they uncoil, dropping to a lower energy level (labeled E1 in the diagram), and emit a radio photon. The process entices other nearby molecules to do the same, and an avalanche of emission ensues, resulting in the bright, monochromatic maser line. Masers rely on an external energy source, such as a nearby, hot star, to pump the molecules back into their excited state (E2), and then the whole process starts again. The first masers to be discovered came from the hydroxl radical (OH), silicon oxide (SiO), and water (H2O). Other masers have been discovered from molecules such as methanol (CH3OH), ammonia (NH3), and formaldehyde (H2CO).