* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electric Current/Electrical Energy
Survey
Document related concepts
Transcript
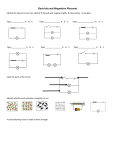
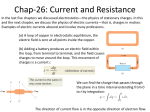
Electric Current/Electrical Energy Current • The rate at which charges pass a given point. The higher the current, the greater the number of charges that pass the point each second. • Measured in Amperes (amps) 2 types of current • Direct current – charges flow in one direction (charges from batteries) • Alternating current – charges continually shift from flowing in one direction to flowing in the reverse direction. (home outlets) Voltage • The potential difference between 2 points in a circuit. • Expressed in volts (v). • (Basically, how much energy is needed to get the charge through the wire) • If an item doesn’t need a lot of electricity to run, it’s low voltage. If it needs a lot, it’s high voltage. Resistance • The opposition to the flow of electric charge. • Resistance is expressed in Ohm’s. • Electrical wiring is very low resistance. Light bulbs, toaster wire is high resistance. • The bigger the wire, the less resistance. The colder the wire, the less resistance. Superconductors • Certain materials, that if cooled enough, will have a resistance of 0 Ohms. • Superconductors also repel magnets Cells • Change chemical or radiant energy into electrical energy. Contains a mixture of chemicals called an electrolyte, which allow charges to flow. • Also contains electrodes, which is the part of the cell where charges enter and exit. • There are 2 kinds of cells – wet cells and dry cells. Wet/Dry Cells • Wet Cell Ex: Car battery • Combine the use of acids as the electrolyte, and metal connectors as electrodes. • Dry Cell Ex: Batteries – • The electrolytes are solid or pastelike Thermocouples • Converts thermal energy into electrical energy. • Thermocouples do not generate a lot of energy Photocells • Converts light energy into electrical energy. • When light shines on the photocell, electrons gain energy to move between atoms. The electrons move through a wire to provide energy to power a device. • Ex: Solar panel