* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrical Properties
Photon polarization wikipedia , lookup
Density of states wikipedia , lookup
Electric charge wikipedia , lookup
Thermal conductivity wikipedia , lookup
Condensed matter physics wikipedia , lookup
Electron mobility wikipedia , lookup
Superconductivity wikipedia , lookup
Thermal conduction wikipedia , lookup
Electrostatics wikipedia , lookup
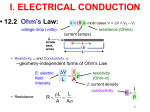
Module-14 Electrical properties Contents 1) Electrical conduction 2) Semi conductivity - Super conductivity 3) Electrical conduction in ionic ceramics and polymers 4) Dielectric behavior 5) Ferroelectricity - Piezoelectricity Introduction Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important role because of their physical properties. Prime physical properties of materials include: electrical properties; thermal properties; magnetic properties; and optical properties. The electrical behaviors of engineering materials are diverse, and so are their uses in electrical applications. Electrical conduction Materials are classified based on their electrical properties as conductors, semiconductors and insulators. New to this group is super conductors. Electrical conductivity of a material is defined in terms of ease of charge flow through it. Charge that flows comprised of either electrons, ions, charged holes, and their combinations. Ohm’s law relates the current and applied voltage: V = IR where V – applied voltage (volts) I – current (amperes) R – resistance (ohms) Electrical conduction (contd…) Material’s electric resistance is NOT an intrinsic-property i.e. it depends on object geometry. Electrical resistivity, defined as follows, is an intrinsic property, inverse of which called conductivity. A – cross sectional area l – length of the object RA l 1 l RA Either conductivity / resistivity can be used to classify materials. Classification – Electrical conductivity Conductivity of solid engineering material is observed to vary over 27 orders of power. Based on their conductivity, materials are classified as: conductors, semiconductors, and insulators. Conductors > 107 (Ω-m)-1 Semiconductors 10-6 - 104 (Ω-m)-1 Insulators < 10-10(Ω-m)-1 Conduction – Flow of charge Power loss due to conduction is given by P = VI = I2R When this is combined with conductivity equation, I A V l J E It (J – current density) is also expressed as J n.q.v It results in n.q. Charge carriers - Conductivity From the last equation, electrical conductivity of a material can be controlled by (i) controlling number of charge carriers, n or (ii) controlling the mobility of the carriers, μ. Mobility is important for metals or electrical conductors, whereas number of carriers is important for semi-conductors and insulators. • for metals : ne .qe . • for semiconductors : • for ionic materials : n.q. total n electronic e n.q. p ionic Genesis of electrical conduction It is known that electrons are prime charge carriers. Electrons in metals are arranged into shells and sub-shells in accordance with Pauli exclusion principle with distinct energy state/level. In metals many electrons will have same energy state, leading to formation of energy bands. The electrons not involved in bonding remain in what is called the core band, whereas the valence electrons that form the electron gas enter into the valence band. The remaining un-filled orbitals form higher-energy bands, called conduction band. It is the conduction band that gives metals and alloys the ability to freely conduct electrons. Energy band structures The energy corresponding to the highest filled state at 0 K is called the Fermi energy, Ef. Four types of band structures are possible at 0 K as shown in the following figure. Energy band structures (contd…) Band structures (1) and (2) are found in metals / conductors. Band structures (3) and (4) are distinguished by the size of energy band gap. Narrower energy band gap i.e. size < 2 eV, is found in semiconductors, while the broader energy band gap i.e. size > 4 eV, is found in insulators. Fermi energy for the last two band structures lies within the band gap near its center. Metals have high conductivities because of the large number of free electrons that have been excited into empty states above the Fermi energy. Mathiessens rule - Resistivity Lattice vibrations and phonon scattering play a role in disrupting the mean free path of electrons. In addition, crystalline defects and impurity atom affect the conductivity. These scattering mechanisms act independently on one another. Thus the effective resistivity of metals can be represented as follows: thermal residual With increase of temperature, thermal vibrations increase so the resistivity, and vice versa. In the same manner, with increase of either defects or impurities, resistivity increases. For pure metals, the resistivity approaches zero at absolute zero temperature. Superconductivity Some metals lose all resistivity abruptly and completely at some low temperatures, above 0 K. - phenomenon is called superconductivity, and the materials that exhibit it are called superconductors. The temperature at which the resistivity vanishes is called the critical transition temperature, Tc. Many metals, solid-solution alloys, some ceramics and intermetallic compounds exhibit superconductivity. Ex.: Ti, V, Zn, W, Al, Hg, NbTi, Nb3Sn, MgB2, La-Sr-Cu oxide, YBa2Cu3O7-x, carbon nanotubes, etc. BCS (Bardeen, Cooper and Schrieffer) theory explains that the super conductivity is caused by electron-lattice interaction and that the superconducting electrons consist of paired ordinary electrons called a Cooper pair. Semiconductivity Electrical properties of semiconductors are unique, in the sense that their electrical properties are extremely sensitive to even minute concentrations of impurities. Two kinds of semiconductors – intrinsic and extrinsic. For intrinsic semiconductors, their electrical behavior is based on inherent electronic structure of the pure material. On the other hand, if the electrical properties are dominated by impurities, they are called extrinsic semiconductors. In semiconductors, the valence and conduction bands do not overlap as in metals, but they possess enough electrons in the valence band those can be promoted to the conduction band at a certain temperature. Intrinsic semiconduction Conduction is due to promoted electrons, and charged hole left behind by these electrons. This occurs at elevated temperatures. At still higher temperatures, the concentration of thermally excited electrons in the conduction band becomes so high that the semiconductor behaves more like a metal. Ex.: Si, Ge, Sn, Pb, etc. Extrinsic semiconduction The charge carrier density can also be increased by adding impurities of either higher or lower valence to intrinsic semiconductors. This addition of impurities is known as doping, and impure atoms in the element are called donor atoms. n-type semiconductor uses higher valence elements as donors, while p-type semiconductors uses lower valence elements. Donor atoms increases number of charge carriers in form negatively charged electrons (n-type) or positively charged holes (p-type). Doping also results in altering the Fermi energy level, and its exact position (Ef) is a function of both temperature and donor concentration. n-type semiconduction Fermi level (Ef) of n-type extrinsic semiconductor is higher than that of intrinsic semiconductor (Ef1) i.e. it shifted upward in the band gap, to within the vicinity of the donor state. Ex.: Pentavalent substitutional atoms like P, As, or Sb are used as donors. p-type semiconduction Fermi level (Ef) of p-type extrinsic semiconductor is lower than that of intrinsic semiconductor (Ef1) i.e. it shifted downward in the band gap, to within the vicinity of the donor state. Ex.: Trivalent substitutional atoms like B, Al, Ga, In, Th are used as donors. Electrical conduction in ionic ceramics Charge can also be conducted via ions - called ionic conduction. This may occur either in conjunction with or separately from electronic conduction. Several types of compounds show exceptionally high ionic conductivity. Such phases fall into three broad categories: halide and chalcogenides of silver and copper; oxides with β-alumina structure; and oxides of fluorite structure. Ex.: La2CuO4 (Tc = 30 K), YBC compounds – yttrium doped perovskite structure, YBa2Cu3O7 (Tc = 92 K). By properly engineering the point defects, it is possible to convert ceramics into semiconductors. Ex.: Indium tin oxide (ITO). Electrical conduction in polymers Polymers are, in general, insulators. They can be made conductors in two ways: (1) introducing an additive to the polymer to improve conductivity, and (2) creating polymers with inherent conductivity. (1) Adding ionic compound or Introducing conductive fillers such as carbon black. (2) Inherent conductivity by doping. Ex.: polyparaphynylene, polypyrole, polyaniline, acetal polymers. Some other polymers such as polyphthaocyanine can be cross-linked by special curing processes to raise its conductivity. Dielectric behavior concept Dielectric is a material separating two charged bodies. For a material to be a good dielectric, it must be an electrical insulator. Dielectric materials are used in capacitors, devices used to store the electric energy. Energy stored, Q, between two plates having area, A, charged by voltage, V, separated at a distance, l, in vacuum is given by Q CV C 0 A l where ε0 – permittivity of vacuum (8.85 X 10-12 F/m) For a dielectric material, ε = εr ε0 εr value is always greater than ‘1’, as a result of polarization. Capacitor concept Polarization (P) is orientation of permanent or induced dipoles under externally applied electric field. It results in charge density increase above that for a vacuum because of the presence of the dielectric material. Surface charge density for a capacitor D P 0 Polarization Four types of polarization: electronic, ionic (atomic), orientation, and space charge (interfacial). Electronic polarization arises because the center of the electron cloud around a nucleus is displaced under an applied electric field. Ionic polarization occurs in ionic materials because an applied field acts to displace cations in the direction of the applied field while displacing anions in a direction opposite to the applied field. Orientation polarization can occur in materials that possess permanent electric dipole moments. These permanent dipoles tend to become aligned with the applied electric field, but entropy and thermal effects tend to counter this alignment, thus it is highly temperature dependent. Space charge polarization results from the accumulation of charge at structural interfaces in heterogeneous materials. Such polarization occurs when one of the phases has a much higher resistivity than the other. Power loss During the use, polarization subjected to an alternating electric field depends upon the ease with which the permanent or induced dipoles can reverse their alignment. The time required for dipole reversal is called the relaxation time, and its reciprocal is called the relexation frequency. As the frequency of the applied electric field approached the relaxation frequency, the polarization response increasingly lags behind the applied field. The reorientation of each type of dipole is opposed by internal friction, which leads to heating in the sample and power loss. The power loss depends on the degree to which the polarization lags behind the electric field. This is also called dielectric loss, and a low dielectric loss is desired at the frequency of utilization. Dielectric strength At high enough frequencies, the dielectric will experience electrical breakdown initiated by the field-induced excitation of a number of electrons into the conduction band, and the insulator become a conductor. The magnitude of the electric field required to cause dielectric breakdown is called the dielectric strength or breakdown strength. Many ceramics and polymers are utilized as insulators and in capacitors. For example: glass, porcelain, stealite, mica. These have dielectric constants within the range of 6-10. Typical applications: electrical insulation, switch bases, light receptacles. Dielectric constant for most polymers lies in the range of 2-5, less than that for ceramics, since the later exhibit greater dipole moments. Typical applications: insulation for wires, cables, motors, generators, some capacitors. Ferro-electricity Ferro-electricity is defined as the spontaneous alignment of electric dipoles by their mutual interaction in the absence of an applied electric field. It arises from the fact that the local field increases in proportion to the polarization. Thus, ferro-electric materials must possess permanent dipoles. Ex.: BaTiO3, Rochelle salt (NaKC4H4O6.4H2O), potassium dihydrogen phosphate (KH2PO4), potassium niobate (KNbO3). These materials have extremely high dielectric constants at relatively low applied field frequencies. Thus, capacitors made from ferro-electric materials are smaller than capacitors made of other dielectric materials. Piezo-electricity Piezo-electricity, or pressure electricity, is defined as polarization induced by the application of external force. Thus by reversing the direction of external force, direction of the field can be reversed i.e. the application of an external electric field alters the net dipole length and causes a dimensional change. Hence piezo-electric materials are useful as transducers – devices that convert mechanical stress into electrical energy and vice versa. Application for these materials includes microphones, ultrasonic generators, sonar detectors, and mechanical strain gauges. Ex.: Barium titanate, lead titanate, lead zirconate (PbZrO3), ammoinium dihydrogen phosphate (NH4H2PO4), and quartz.