* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download R2-06-04
Power engineering wikipedia , lookup
History of electric power transmission wikipedia , lookup
Switched-mode power supply wikipedia , lookup
Electrification wikipedia , lookup
Distribution management system wikipedia , lookup
Thermal runaway wikipedia , lookup
Power over Ethernet wikipedia , lookup
Power electronics wikipedia , lookup
Voltage optimisation wikipedia , lookup
Power MOSFET wikipedia , lookup
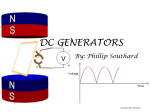
Alternating current wikipedia , lookup
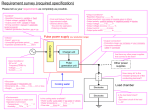
CNSE Technology Management Non-Confidential Description: CNSE Case #R2-06-04 Invention Title: Direct Powering of Implantable Bio-medical Devices with ThermoElectric Generator Keywords: Power generation, thermo-electrics, thermal energy, thermo-electric generator, nano-generator, bio-devices, implantable device, pacemaker, living organism, patient data chip Intellectual Property Status: Invention Disclosure Inventor(s): Dr. Pradeep Haldar; Dr. Harry Efstathiadis Invention Description: A thermoelectric generator is a device that uses small temperature differences to generate electricity. For this application, the device must be as small and efficient as possible; and utilizing nanoengineering techniques allows us to do that. Two dissimilar materials, p-type (boron carbide layers) and n-type (silicon and silicon carbide multi-layers), are layered between different conductors. Each layer is approximately 10 nanometers. Using superlatticing technology (multi-layering films), efficiency of a nanogenerator is increased four times the current technology using bulk materials. This increase in efficiency is estimated to decrease the nanogenerator cost by half. The nanogenerator needs only a very slight temperature gradient in order to create 10-40 microwatts, the amount needed to power a pacemaker. Additionally, the device will include a polarity reverser, so that if the person travels to a hot climate, the generator will not reverse to become a cooling device. A voltage polarity reverser and voltage control circuits are used to ensure that power is generated regardless of whether Tc (skin temperature) is higher or lower than Th (inner body temperature). The implant device comprises of a biocompatible primary case housing the bio-generator, a polarity reversal unit, a voltage regulator, a power monitor, and the implantable device data-chip, pacemakers, stimulators, drug infusion pumps etc. Since the bio-generator and the associated control units will be included within the implantable device enclosure, no FDA class III approval is needed for the bio-generator and the associated electronics, i.e. no clinical trials to human subjects and animals needed. CNSE Technology Management [email protected]