* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organometallic Reactions and Catalysis
Survey
Document related concepts
Kinetic resolution wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Metal carbonyl wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Transcript
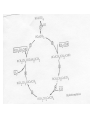
Organometallic Reactions and Catalysis Chapter 14 Gain or Loss of Ligands • CO dissociation – In many cases to add another ligand. – Dissociative and associative mechanisms – More complicated reactions. • Dissociation of phosphine (steric effects) – cis-Mo(CO)4L2 + CO Mo(CO)5L + L – Figure 14-1 and Table 14-1 (Article) – Rate dependence on cone angle and other factors. • Reaction follows the first-order rate law. Oxidative Addition (OA) • Increases the coordination number and the oxidation state of the metal. • OA reactions of square-planar d8 complexes. – trans-Ir(CO)Cl(PEt3)2 (Figure 14-3) • Changes in CN and oxidation state • Reactions may occur between ligands due to close proximity. Reductive Elimination (RE, reverse of OA) • Decrease in coordination number and oxidation state of the metal. OA (-C5H5)2TaH + H2 (5-C5H5)TaH3 RE • RE reaction rates are also affected by ligand bulk. How? (Table 14-2) Nucleophlic Displacement (attack by a Lewis base) • A strong nucleophile would be a ligand with strong electron-donating character. • Organometallic complexes can behave as nucleophiles in displacement reactions (especially negativelycharged complexes). [Co(CO)4]- + RX RCo(CO)4 + XRCo(CO)4 + CO R(C=O)Co(CO)4 (acyl complex) R(C=O)Co(CO)4 +R’OH R(C=O)OR’ + HCo(CO)4 (generates the ester from an alcohol). Modification of Ligands • Insertion – a molecular fragment appears to insert itself into a metal-ligand bond. – Many reaction mechanism can be complicated. – 1,1-insertion (both bonds are made to the same atom). • illustrate – 1,2-insertion (bonds to the inserted molecule are made to adjacent atoms in that molecule). • illustrate Insertion of Ligands • How is CO inserted in the complex shown previously (1,1-insertion)? – Work through this and understand. • 1,2-insertions Hydride Elimination • Transfer of a hydrogen atom from a ligand to a metal. – elimination is the most common type. • position on the alkyl ligand. • Stability – Alkyl complexes that lack hydrogens are more stable. – Coordinatively saturated complexes containing alkyl ligands are also more stable. Abstraction • Removal of a substituent from a ligand in which the coordination number of the metal does not change (can be removed by an acid). Organometallic Catalysts (hydroformylation) • Converting terminal alkenes into other organic products. – (oxo process) H and HCO are formally added across a double bond. • Show reaction • Largest-scale industrial process that is homogeneous • Mechanism was suggested by Heck and Breslow in 1961. – Examine each step in the cycle and characterize the reaction according to type. • 18-,16-electron cycling is common. Comments on the Hydroformylation Mechanism • CO pressure has to be controlled carefully. Why? • Rate-determining step is the insertion of the olefin (alkene). • Main purpose of the reaction is to produce butanal from propene. CH3CH=CH2CH3CH2CH2CHO Union Carbide Hydroformylation Process • Contain Rh and bulky phosphine groups. How will this affect the mechanism? – (Ph3P)3Rh(CO)H • In many cases, the linear/branched ration needs to high. • The catalyst is also water-soluble. Hydrogenation of Alkenes • Wilkinson’s catalyst – Show reaction (alkenes and alkynes) • Show mechanism and discuss – Step 9 is slow, the sequence 123 is favored. – The rate determining step is insertion, 4. • The catalyst hydrogenizes terminal and internal olefins. • Examine Table 14-3. Hydrogenation Catalyst • Selective hydrogenation can be observed if the ligand contains multiple double bonds. • Another hydrogenation catalyst, (PPh3)2Rh(CO)H, is very selective toward hydrogenation of only terminal olefins. • Asymmetric hydrogenation – If the catalyst, [L2RHS2]+, bears an optically active diphosphane, prochiral unsaturated molecules can be hydrogenated to chiral products (enantiomeric selectivity). • L-Dopa (treatment of Parkinson’s disease). Alkene Metathesis • Demonstrate – Propene and 1-butene (what are the 4 new products that may form from methathesis?) • Ring-opening metathesis (ROM) • Chauvin mechanism is most widely accepted. – Involved a carbene complex – The carbene reacts with an alkene to form a metallocyclobutane intermediate. The intermediate can either revert to reactants or form new products. – Schrock metathesis catalysts are most effective and the most studied (available commercially). • Ring-closing methathesis (page 545) Heterogeneous Catalysis • Used much more extensively in industry than homogeneous catalysts. – Robust at high temperatures. – Easy to separate out the catalyst. Composition of Heterogeneous Catalysts • Uniform – bulk of the high-surface area serves as the catalyst. – ZSM-5 (zeolite) • Multiphase – highsurface-area material serves as a support for the active catalyst. – Pt/Re on alumina Surface Ligands • In many cases, the nature of the surface ligand is inferred by comparison of IR spectra with those of organometallic or inorganic complex. – Terminal and bridging CO. Surface-Sensitive Techniqus • Temperature-programmed desorption (mass spectroscopy). • Photoelectron spectroscopy (XPS and Auger) • Low-Energy Electron Diffraction (LEED) • Scanning Tunneling and Atomic Force Microscopies. • Vibrational Techniques (RAIRS and HREELS). • Many othes. Catalytic Steps • Many parallels can be drawn in comparison to organometallic mechanisms studied previously. • Chemisorption and physisorption – Similar to interactions present in complexes with low oxidation states. – Physisorption and chemisorption. Catalytic Steps • Similar to homogeneous catalysis, there is also a balance between strong enough adsorption for the reaction to occur and weak enough desorption that the species can be removed for further reactions. – HCOOH CO + H2O • (on a metal surface) Diversity of Sites • Real surfaces possess a large diversity of surface types. Each surface type may have a different reactivity and/or produce different products. – Lower selectivity. – Most reactive sites. Examples of Heterogeneous Catalysts • Hydrogenation of alkenes on metal surfaces. – H2 is dissociatively chemisorbed – Ethylene is associated – Hydrogen adds to produce an alkyl species – Another hydrogen atom coordinates and ethane leaves. – Actual species produced Ziegler-Natta Polymerization • TiCl4 + Al(C2H5)3 – A titanium alkyl complex is produce. – Ethylene or propylene associates and inserts into the titanium-carbon bond. – The 1,2-insertion continues. – Mechanism has proved difficult to understand. In Miessler and Tarr Fundamental Studies of Hydrocarbons on Platinum Surfaces • The techniques used. – Reflection-absorption infrared spectroscopy. – Auger electron spectroscopy – Temperature-programmed desorption/reaction spectroscopy. – Others as needed. Reflection-Absorption Infrared Spectroscopy (RAIRS) • The dynamic dipole moment must have a component normal to the surface to be visible. • The intensity of the vibration signature reveals orientation information. • Position of the signature indicates identity of species on the surface. _ q(t) + qcos + _ = + _ + _ + _ _ + qsin Absorbance A Typical RAIRS Spectrum c b a 1300 1350 1400 1450 1500 1550 1600 2400 2500 2600 2700 2800 2900 3000 3100 Frequency (cm-1) The Labeling Study 0.0006 0.00005 Absorbance Absorbance CD2HCD2(CH2)2CD2CD2H n-C8H18 x 15 CH3CH2(CD2)2CH2CH3 x4 CD3(CH2)6CD3 1440 CH3(CH2)4CH3 2400 2500 2600 2700 2750 2800 Frequency (cm-1) 2700 2800 -1 Frequency (cm ) 2900 3000 2850 2900 2950 Cyclic C8 Systems on Pt(111) . . 250-325 K cyclooctene cyclooctenediyl C8H12 C8H14 5 37 532 K 1,3-cyclooctadiene C8H12 325 -375 K . 250-300 K 1,5-cyclooctadiene C8H12 . or cyclooctadienediyl C8H10 325-375 K cyclooctatetraene 1,5-dihydropentalene K 325-375 K 00 3 522 cyclooctatetraene C8H8 175-225 K bicyclo[3.3.0]2-octene C8H12 1,5-dihydropentalene C8H8 Pentalene C8H6 Stable to ~500 K, decomposes to yield predominantly surfacebound carbon, hydrogen desorbs. 1,4-Cyclohexadiene on Pt(111) 0.0003 Absorbance 100 K Hdistal Hdistal 150 Hproximal 200 f 250 300 350 2400 2500 Hproximal 2600 2700 2800 2900 -1 Wavenumber (cm ) 3000 3100