AOW- Time Travel
... states,” he says, “you can violate the Heisenberg uncertainty principle.” Heisenberg's uncertainty principle says certain pairs of things can't be measured accurately at the same time. Basically, the better you know the position of a particle, the less you know its momentum, and vice versa. "But if ...
... states,” he says, “you can violate the Heisenberg uncertainty principle.” Heisenberg's uncertainty principle says certain pairs of things can't be measured accurately at the same time. Basically, the better you know the position of a particle, the less you know its momentum, and vice versa. "But if ...
fund_notes_up2 (new_version)
... Physicists since Einstein have been trying to understand gravity, and to reduce the expressions for the four forces of the universe to a single equation. According to Leonard Susskind, by the 1950s, Richard Feynman, Julian Schwinger, SinItiro Tomanaga and Freeman Dyson had laid the foundation for a ...
... Physicists since Einstein have been trying to understand gravity, and to reduce the expressions for the four forces of the universe to a single equation. According to Leonard Susskind, by the 1950s, Richard Feynman, Julian Schwinger, SinItiro Tomanaga and Freeman Dyson had laid the foundation for a ...
On the Ionization Energy of the Outer Electrons of Atoms and Their
... rigorous quantitative theory that it is not more than a theory of the hydrogen and helium atoms and of some other elementary systems” [1]. The well developed approximation methods allow us to calculate the energies of the stationary states of complex atoms with certain accuracy, however, numerical c ...
... rigorous quantitative theory that it is not more than a theory of the hydrogen and helium atoms and of some other elementary systems” [1]. The well developed approximation methods allow us to calculate the energies of the stationary states of complex atoms with certain accuracy, however, numerical c ...
Document
... • The splitting of spectral lines due to the splitting of the energy terms of atoms in a magnetic field is called the Zeeman effect. ...
... • The splitting of spectral lines due to the splitting of the energy terms of atoms in a magnetic field is called the Zeeman effect. ...
Physics 125a – Problem Set 5 – Due Nov 12,... Version 3 – Nov 11, 2007
... Section 5. Finally, some real quantum mechanics! v. 2: Provide result for transmission as a function of wavevector in (5b). More specificity on how to do plot. v. 3: In (5b), had mistakenly written k1 and k2 as if the well were at −V0 and the potential was zero elsewhere, instead of what is given, w ...
... Section 5. Finally, some real quantum mechanics! v. 2: Provide result for transmission as a function of wavevector in (5b). More specificity on how to do plot. v. 3: In (5b), had mistakenly written k1 and k2 as if the well were at −V0 and the potential was zero elsewhere, instead of what is given, w ...
Erwin Schroedinger gained inspiration
... For a given element, the emission lines and the absorption lines occur at the same frequency. This is where quantum mechanics comes in. Here’s the basic idea (which was the product of Niels Bohr, Erwin Schroedinger, and Verner Heisenberg). The atom has a minimum energy state which is called its gro ...
... For a given element, the emission lines and the absorption lines occur at the same frequency. This is where quantum mechanics comes in. Here’s the basic idea (which was the product of Niels Bohr, Erwin Schroedinger, and Verner Heisenberg). The atom has a minimum energy state which is called its gro ...
a project report - India Study Channel
... scattering, the stable states of Bohr atom, the emission of spectral lines etc., but the difficulty arises when we wish to follow the flight of a single electron or any other material particle. The wave packet associated with material particle dissipates in a course of time, so that it cannot repres ...
... scattering, the stable states of Bohr atom, the emission of spectral lines etc., but the difficulty arises when we wish to follow the flight of a single electron or any other material particle. The wave packet associated with material particle dissipates in a course of time, so that it cannot repres ...
Electron Configuration Notes
... • electrons move around nucleus in orbits similar to how planets orbit the sun • energy levels for electrons are quantized Major developments that put Bohr’s Model into question: Einstein: Light energy exhibits properties of matter. Matter and energy are different forms of the same thing. De Broglie ...
... • electrons move around nucleus in orbits similar to how planets orbit the sun • energy levels for electrons are quantized Major developments that put Bohr’s Model into question: Einstein: Light energy exhibits properties of matter. Matter and energy are different forms of the same thing. De Broglie ...
2 - Physics at Oregon State University
... • Also called “spin”, or spin angular momentum, or S • It’s a “degree of freedom”, or quantum number: a “state” the particle has • Does interact with magnetic fields like L, but not continuous! • NOT a physical rotation • INTRINSIC property – like charge and rest mass! We have no model for what “mak ...
... • Also called “spin”, or spin angular momentum, or S • It’s a “degree of freedom”, or quantum number: a “state” the particle has • Does interact with magnetic fields like L, but not continuous! • NOT a physical rotation • INTRINSIC property – like charge and rest mass! We have no model for what “mak ...
Assignment Chemistry Class XI (2016-17)
... buoyant force, work done by torque, pressure energy, mass per unit area. 15. If (P + a/V2) (V – b)=RT, where the symbols have their usual meanings, then (a/b) has a dimension of………. 16. Force(F) and density(d) are related as F = α/ (β+√d) (i)then the dimensions of α are………(ii) then the dimensions of ...
... buoyant force, work done by torque, pressure energy, mass per unit area. 15. If (P + a/V2) (V – b)=RT, where the symbols have their usual meanings, then (a/b) has a dimension of………. 16. Force(F) and density(d) are related as F = α/ (β+√d) (i)then the dimensions of α are………(ii) then the dimensions of ...
Chapter 5
... Electrons were discovered in 1897 by Sir J. J. Thomas Electrons have the following characteristics: Travel from a cathode (electron emitter) in straight lines. Cause a piece of metal foil to become hot by striking it for a period of time. Deflected by magnetic and electric fields showing that they h ...
... Electrons were discovered in 1897 by Sir J. J. Thomas Electrons have the following characteristics: Travel from a cathode (electron emitter) in straight lines. Cause a piece of metal foil to become hot by striking it for a period of time. Deflected by magnetic and electric fields showing that they h ...
Qualifying Exam for Graduate Students – Fall 2008
... (d) Repeat the previous steps for Maxwell’s equations in a linear dielectric (with no free charge or current) to get a new wave equation for E. Hint: use D = r0E. What do you obtain for v in this case? How does your result relate to what first year physics undergrads are often taught, that the spe ...
... (d) Repeat the previous steps for Maxwell’s equations in a linear dielectric (with no free charge or current) to get a new wave equation for E. Hint: use D = r0E. What do you obtain for v in this case? How does your result relate to what first year physics undergrads are often taught, that the spe ...
Document
... the angular momentum ⇒ zero point energy can be zero! 3 Two wavefunctions with different quantum numbers can have the same energy. For example wavefunctions with ml = 1 and −1 have the same energy, ћ2/2I. This is known as degeneracy. Example: Let us treat the π-electrons in benzene as particles of m ...
... the angular momentum ⇒ zero point energy can be zero! 3 Two wavefunctions with different quantum numbers can have the same energy. For example wavefunctions with ml = 1 and −1 have the same energy, ћ2/2I. This is known as degeneracy. Example: Let us treat the π-electrons in benzene as particles of m ...
Chemistry in Four Dimensions
... The reluctance to abandon dogmatic theory often results in the introduction of secondary ad hoc explanations to cover up any cracks in the theory, as they occur. A prime example occurs in the quantum theory of elemental periodicity. Based on the wave-mechanical ordering of electronic energy levels i ...
... The reluctance to abandon dogmatic theory often results in the introduction of secondary ad hoc explanations to cover up any cracks in the theory, as they occur. A prime example occurs in the quantum theory of elemental periodicity. Based on the wave-mechanical ordering of electronic energy levels i ...
Lecture 3
... In the preceding lectures we found that we can some how calculate the wave function or the probability Ψ(r,t) at all points at each instant of time then the statistical moments such as average value, variance, standard deviation of all other physically observable quantities such as position, momentu ...
... In the preceding lectures we found that we can some how calculate the wave function or the probability Ψ(r,t) at all points at each instant of time then the statistical moments such as average value, variance, standard deviation of all other physically observable quantities such as position, momentu ...
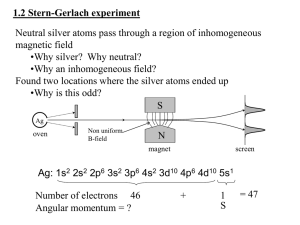
Section Three: Light and Matter
... first single slit is required only a double slit). The arrangement above is equivalent to two rocks being thrown into a lake, or when sound from two loudspeakers interferes. These two slits have circular waves leaving them because of the diffraction effect. Using the diagrams we can see how an inter ...
... first single slit is required only a double slit). The arrangement above is equivalent to two rocks being thrown into a lake, or when sound from two loudspeakers interferes. These two slits have circular waves leaving them because of the diffraction effect. Using the diagrams we can see how an inter ...
Tunneling and the Vacuum Zero
... of the stochastic processes theory is the study of escape rates over a potential barrier. The theoretical approach, first proposed by Kramers [1], has many applications in chemistry kinetics, diffusion in solids, nucleation [2], and other phenomena [3]. The essential structure of the escape process ...
... of the stochastic processes theory is the study of escape rates over a potential barrier. The theoretical approach, first proposed by Kramers [1], has many applications in chemistry kinetics, diffusion in solids, nucleation [2], and other phenomena [3]. The essential structure of the escape process ...
B.Sc. (General Sciences)
... 8. To construct the RC differentiator and study the response to time varying signal. 9. To construct the RC integrator and study the response to time varying signal. 10. To study the I-V characteristics of (a) resistances (Ohm and mega ohm range) (b) Tungsten bulb (c) diode or solar cell. (d) zener ...
... 8. To construct the RC differentiator and study the response to time varying signal. 9. To construct the RC integrator and study the response to time varying signal. 10. To study the I-V characteristics of (a) resistances (Ohm and mega ohm range) (b) Tungsten bulb (c) diode or solar cell. (d) zener ...
Photoelectric-Effect-and-Nuclear-2
... Okay? Got it? Now you make a small hole in the ball. Having done that, you now look into the hole – what do you see? Well, the whole looks black. The inside of the ball looks black, even though we know that it is actually white. So what’s the deal? The interior of the ball behaves as if were a black ...
... Okay? Got it? Now you make a small hole in the ball. Having done that, you now look into the hole – what do you see? Well, the whole looks black. The inside of the ball looks black, even though we know that it is actually white. So what’s the deal? The interior of the ball behaves as if were a black ...
Document
... The brilliant red color seen in fireworks displays is due to 4.62 x 1014 s-1 strontium emission. Calculate the wavelength of the light emitted. ...
... The brilliant red color seen in fireworks displays is due to 4.62 x 1014 s-1 strontium emission. Calculate the wavelength of the light emitted. ...