presentation on power generation from biogas in 2
... The gas from the bottom of the Scrubber enters the packed Column while liquid is collected in the Tank for recalculation. In the II stage of scrubbing, gas is scrubbed with caustic solution in a packed Column. The Column is provided with ceramic rings to have enhanced surface area for mass transfer. ...
... The gas from the bottom of the Scrubber enters the packed Column while liquid is collected in the Tank for recalculation. In the II stage of scrubbing, gas is scrubbed with caustic solution in a packed Column. The Column is provided with ceramic rings to have enhanced surface area for mass transfer. ...
AP gas notes 2010
... Each exerts a specific pressure(called partial pressure) as it would by itself Total Pressure of gas mixture = sum of the partial pressures of each individual gas ...
... Each exerts a specific pressure(called partial pressure) as it would by itself Total Pressure of gas mixture = sum of the partial pressures of each individual gas ...
METHODOLOGY FOR RESIDUAL OXYGEN CALORIMETRY
... Requirement Index (CARI) of the fuel, which can then be mathematically correlated to the Wobbe Index. Differences between the CARI and Wobbe Index values can be cancelled out by the use of calibration gases thus giving this technique the ability to report Wobbe Index values along with CARI. With the ...
... Requirement Index (CARI) of the fuel, which can then be mathematically correlated to the Wobbe Index. Differences between the CARI and Wobbe Index values can be cancelled out by the use of calibration gases thus giving this technique the ability to report Wobbe Index values along with CARI. With the ...
0191 271 0222 Nitrogen (Oxygen Free)
... applicable European Directives and applies to all countries that have translated the Directives in their national laws. Disclaimer of Liability: Details given in this document are believed to be correct at the time of going to press. Whilst proper care has been taken in the preparation of this docum ...
... applicable European Directives and applies to all countries that have translated the Directives in their national laws. Disclaimer of Liability: Details given in this document are believed to be correct at the time of going to press. Whilst proper care has been taken in the preparation of this docum ...
Chemistry- The Gas Phase
... 12.8 In a car engine, gasoline (mostly octane, C8H18) is burned to make CO2(g) and H2O(g). If a gallon of octane is burned in excess O2 at P=1.00 atm and T=180oC, how many L of CO2 are produced? 1 gallon of octane = 3.2 x 103 g. ...
... 12.8 In a car engine, gasoline (mostly octane, C8H18) is burned to make CO2(g) and H2O(g). If a gallon of octane is burned in excess O2 at P=1.00 atm and T=180oC, how many L of CO2 are produced? 1 gallon of octane = 3.2 x 103 g. ...
practice quiz5
... D) various liquids at constant pressure Question 14 Which of the following is not an assumption of the kinetic theory of gases? A) Elasticity refers to the molecules random interactions resulting in no net energy change. B) Gas molecules are viewed as points due to large distances between them. C) ...
... D) various liquids at constant pressure Question 14 Which of the following is not an assumption of the kinetic theory of gases? A) Elasticity refers to the molecules random interactions resulting in no net energy change. B) Gas molecules are viewed as points due to large distances between them. C) ...
AP Ch 5 Gases . ppt
... each other by distances far greater than their own dimensions. The molecules can be considered to be points; that is, they possess mass but have negligible volume. 2. Gas molecules are in constant motion in random directions. Collisions among molecules are perfectly elastic. ...
... each other by distances far greater than their own dimensions. The molecules can be considered to be points; that is, they possess mass but have negligible volume. 2. Gas molecules are in constant motion in random directions. Collisions among molecules are perfectly elastic. ...
Name__________________________ Honors Chemistry Final
... 8. Water and methane (CH4) can react to produce hydrogen gas and carbon dioxide gas. When 0.065 g of methane reacts with excess water, the gaseous products are collected by displacing water in an inverted tube at a pressure of 780 mmHg. The temperature of the water is 17oC. Write the balanced chemic ...
... 8. Water and methane (CH4) can react to produce hydrogen gas and carbon dioxide gas. When 0.065 g of methane reacts with excess water, the gaseous products are collected by displacing water in an inverted tube at a pressure of 780 mmHg. The temperature of the water is 17oC. Write the balanced chemic ...
File
... According to the Vapor Pressure of Four Liquids chemistry reference table, if the pressure on the surface of water in the liquid state is 47.0 kPa, the water will boil at ...
... According to the Vapor Pressure of Four Liquids chemistry reference table, if the pressure on the surface of water in the liquid state is 47.0 kPa, the water will boil at ...
Molecular Mass of a gas
... 2. The reaction: NaHCO3(s) + HCl(aq) → CO2(g) + NaCl(aq) + H2O(l) produces carbon dioxide gas. If the gas were produced at 20°C, where the vapor pressure of water is 18 torr, how many grams of NaHCO3 would be required to produce 60.0 ml of wet CO2? Assume an atmospheric pressure of 763 torr, and an ...
... 2. The reaction: NaHCO3(s) + HCl(aq) → CO2(g) + NaCl(aq) + H2O(l) produces carbon dioxide gas. If the gas were produced at 20°C, where the vapor pressure of water is 18 torr, how many grams of NaHCO3 would be required to produce 60.0 ml of wet CO2? Assume an atmospheric pressure of 763 torr, and an ...
a Gas
... - has neither a shape of its own nor fixed volume. It takes the shape and volume of its container. - Gas mixtures are always homogeneous - Gases are highly compressible. - The molecules of a gas are relatively far away each other. - Individual gas molecules have little interaction with their neighbo ...
... - has neither a shape of its own nor fixed volume. It takes the shape and volume of its container. - Gas mixtures are always homogeneous - Gases are highly compressible. - The molecules of a gas are relatively far away each other. - Individual gas molecules have little interaction with their neighbo ...
CHAPTER 5 REVIEW PACKET – GAS LAWS
... 17. A gas of unknown molecular mass was allowed to effuse through a small opening under constantpressure conditions. It required 105s for 1.00L of the gas to effuse. Under identical experimental conditions, it required 31.0s for 1.00L of O2 to effuse. Calculate the molar mass of the unknown gas. ...
... 17. A gas of unknown molecular mass was allowed to effuse through a small opening under constantpressure conditions. It required 105s for 1.00L of the gas to effuse. Under identical experimental conditions, it required 31.0s for 1.00L of O2 to effuse. Calculate the molar mass of the unknown gas. ...
Chem 30A, Test Review #2
... A mixture of helium, oxygen and nitrogen is stored in a 40.0-L cylinder at a total pressure of 2.50 atm at 20oC. The partial pressure of helium is 460 torr, and that of nitrogen is 950 torr. (a) What is the partial pressure of oxygen in the mixture? (b) How many grams of each gas are present in the ...
... A mixture of helium, oxygen and nitrogen is stored in a 40.0-L cylinder at a total pressure of 2.50 atm at 20oC. The partial pressure of helium is 460 torr, and that of nitrogen is 950 torr. (a) What is the partial pressure of oxygen in the mixture? (b) How many grams of each gas are present in the ...
Gas Laws Practice Test.Ans.Key
... 0.2500 moles of solid zinc and reacts the zinc with an excess of concentrated hydrochloric acid, what is the actual volume (in ml) of hydrogen that should be collected [not the volume of dry gas]? ...
... 0.2500 moles of solid zinc and reacts the zinc with an excess of concentrated hydrochloric acid, what is the actual volume (in ml) of hydrogen that should be collected [not the volume of dry gas]? ...
C:\Users\Jim\Documents\usb key backups\Nov. 17\sch3u\unit 4
... Note just how fast air molecules move on average, even at room temperature (300 K): 500 m/s or 0.5 km/s. Higher temperatures mean higher average speed. However, temperature is proportional to kinetic energy, not speed. Ek = .5 m v2 At the same temperature, molecules with larger molar masses are movi ...
... Note just how fast air molecules move on average, even at room temperature (300 K): 500 m/s or 0.5 km/s. Higher temperatures mean higher average speed. However, temperature is proportional to kinetic energy, not speed. Ek = .5 m v2 At the same temperature, molecules with larger molar masses are movi ...
Elemental Analysis of Semiconductor Gases Using a Gas Exchange
... Ge (RSD) were obtained. In order to assess any memory effect from the GED and the RC sampling system, a pure nitrogen gas blank was introduced immediately after the highest concentration standard and the Ge and As intensities were monitored on the iCAP Qs. After only 6 minutes the Ge and As count ra ...
... Ge (RSD) were obtained. In order to assess any memory effect from the GED and the RC sampling system, a pure nitrogen gas blank was introduced immediately after the highest concentration standard and the Ge and As intensities were monitored on the iCAP Qs. After only 6 minutes the Ge and As count ra ...
NAME…………… - Kcse Online
... (3mks) __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ ____________________________________________ ...
... (3mks) __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ ____________________________________________ ...
Sec. 12.3 Day 2
... Gas Stoichiometry For example, consider the Haber process for the production of ammonia gas… 3 H2(g) + N2(g) → 2 NH3(g) 3 L of H2 react with 1 L of N2 to form 2 L of NH3, and no H2 or N2 is left over. If we know the number of moles of a gaseous substance, we can use the ideal gas law to calculat ...
... Gas Stoichiometry For example, consider the Haber process for the production of ammonia gas… 3 H2(g) + N2(g) → 2 NH3(g) 3 L of H2 react with 1 L of N2 to form 2 L of NH3, and no H2 or N2 is left over. If we know the number of moles of a gaseous substance, we can use the ideal gas law to calculat ...
12.1 Avogadro`s Law and Molar Volume
... 12.1 AVOGADRO’S LAW AND MOLAR VOLUME GAY-LUSSAC’S LAW OF COMBINING VOLUMES When gases react, volumes of gaseous reactants and products of chemical reaction (at equal temperatures and pressures) are in simple whole number ratios. Example: When hydrogen and oxygen come together to produce water, 2 uni ...
... 12.1 AVOGADRO’S LAW AND MOLAR VOLUME GAY-LUSSAC’S LAW OF COMBINING VOLUMES When gases react, volumes of gaseous reactants and products of chemical reaction (at equal temperatures and pressures) are in simple whole number ratios. Example: When hydrogen and oxygen come together to produce water, 2 uni ...
1411-practice exam 2(ch4 5) - Chemistry
... ... 36. Specify how each of the following strong electrolytes ionizes or dissociates into ions upon dissolving in water. a) KNO3 ... doc | 53,8 kB | 6 pages PowerPoint ... 25. The gas pressure in an aerosol can is 1.8 atm at 25C. If the gas is an ideal gas, what pressure would develop in the can if ...
... ... 36. Specify how each of the following strong electrolytes ionizes or dissociates into ions upon dissolving in water. a) KNO3 ... doc | 53,8 kB | 6 pages PowerPoint ... 25. The gas pressure in an aerosol can is 1.8 atm at 25C. If the gas is an ideal gas, what pressure would develop in the can if ...
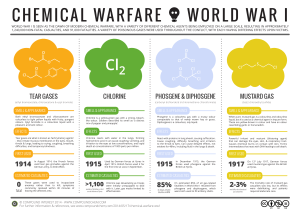
chemical warfare world war i
... Powerful irritant and vesicant (blistering agent) that can damage the eyes, skin, & respiratory tract. Causes chemical burns on contact with skin. Forms intermediates that react with DNA leading to cell death. ...
... Powerful irritant and vesicant (blistering agent) that can damage the eyes, skin, & respiratory tract. Causes chemical burns on contact with skin. Forms intermediates that react with DNA leading to cell death. ...
Oxygen Gas Safety
... Do not lift cylinders by the valve. Use suitable cylinder handling and securing equipment. Do not use oil or grease on valves, fittings, or any other equipment. Always close valves using moderate force only, even when the cylinder is empty. Do not attempt to control the gas flow using the valve. ...
... Do not lift cylinders by the valve. Use suitable cylinder handling and securing equipment. Do not use oil or grease on valves, fittings, or any other equipment. Always close valves using moderate force only, even when the cylinder is empty. Do not attempt to control the gas flow using the valve. ...
Integrated Gasification Combined Cycles
... • Coal is combined with oxygen in the gasifier to produce the gaseous fuel, mainly hydrogen and carbon monoxide. • The gas is then cleaned by a gas cleanup process. • After cleaning, the coal gas is used in the combustion turbine to produce electricity. ...
... • Coal is combined with oxygen in the gasifier to produce the gaseous fuel, mainly hydrogen and carbon monoxide. • The gas is then cleaned by a gas cleanup process. • After cleaning, the coal gas is used in the combustion turbine to produce electricity. ...
Coal gas

Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.Coal gas contains a variety of calorific gases including hydrogen, carbon monoxide, methane and volatile hydrocarbons together with small quantities of non-calorific gases such as carbon dioxide and nitrogen.Prior to the development of natural gas supplies and transmission systems (during the 1940s and 1950s in the US and the late 1960s and 1970s in the UK), virtually all fuel and lighting gas used in both the United States and Great Britain was manufactured from coal. Gas was often supplied to households via a municipally owned piped distribution system.Originally created as a by-product of the coking process, its use developed during the 19th and early 20th centuries tracking the industrial revolution and urbanization. By-products from the production process included coal tars and ammonia, which were important chemical feedstock for the dye and chemical industry with a wide range of artificial dyes being made from coal gas and coal tar. Facilities where the gas was produced were often known as a manufactured gas plant (MGP) or a gasworks.The discovery of large reserves of natural gas in the North Sea off the UK coast during the early 1960s led to the expensive conversion or replacement of most of the nation's gas cookers and gas heaters, with the exception of Northern Ireland, from the late 1960s onwards.The production process is distinct, both physically and chemically, from that used to create a range of gaseous fuels known variously as manufactured gas, syngas, hygas, Dowson gas, and producer gas. These gases are made by partial combustion of a wide variety of feed stocks in some mixture of air, oxygen, or steam, to reduce the latter to hydrogen and carbon dioxide although some destructive distillation may also occur.