Class 23_270_11
... goes through either slit#1 or slit#2 must be wrong. Electron must be going through both slits at the same time !!! to exhibit interference ...
... goes through either slit#1 or slit#2 must be wrong. Electron must be going through both slits at the same time !!! to exhibit interference ...
class slides for Chapter 38
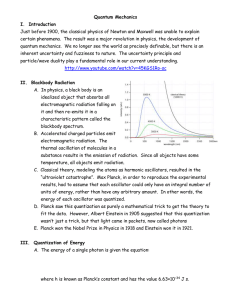
... f0 or, if the wavelength is greater than the corresponding cutoff wavelength l0 =c/f0. This is so no matter how intense the incident light is. The electrons within the target are held there by electric forces. To just escape from the target, an electron must pick up a certain minimum energy f, where ...
... f0 or, if the wavelength is greater than the corresponding cutoff wavelength l0 =c/f0. This is so no matter how intense the incident light is. The electrons within the target are held there by electric forces. To just escape from the target, an electron must pick up a certain minimum energy f, where ...
Class 22
... Which slit did this photon go through? If one slit: Get single slit pattern (i.e. no interference) Like this: or this: The sum of the two: But not like this: But: that photon is part of the two slit interference pattern. The probability pattern of where it lands is described by the 2 slit interfere ...
... Which slit did this photon go through? If one slit: Get single slit pattern (i.e. no interference) Like this: or this: The sum of the two: But not like this: But: that photon is part of the two slit interference pattern. The probability pattern of where it lands is described by the 2 slit interfere ...
Electromagnetic Waves In this lecture Waves Speed of
... • X-ray photons are characterised by energy • Planck developed relationship between energy and frequency ...
... • X-ray photons are characterised by energy • Planck developed relationship between energy and frequency ...
Copenhagen Interpretation (of quantum physics)
... The key concept is the so-called ‘collapse of the wave function’, In seeking to explain how an entity such as a photon or an electron could ‘travel as a wave but arrive as a particle’, Bohr and his colleagues said it was the act of observing the wave that made it ‘collapse’ to become a particle… But ...
... The key concept is the so-called ‘collapse of the wave function’, In seeking to explain how an entity such as a photon or an electron could ‘travel as a wave but arrive as a particle’, Bohr and his colleagues said it was the act of observing the wave that made it ‘collapse’ to become a particle… But ...
Liad Elmelech 7.1-7.3 The Nature of Light, Atomic Spectroscopy
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
Case 2 - Nikhef
... From the detector counts deduce again the probabilities P1 and P2 To avoid confusion use single electrons: one by one! ...
... From the detector counts deduce again the probabilities P1 and P2 To avoid confusion use single electrons: one by one! ...
Quantum Potpourri
... keep saying to yourself, if you can possibly avoid it, 'But how can it possibly be like that?' because you will go down the drain into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. [Richard Feynman] • Any one who is not shocked by quantum mechanics has not fu ...
... keep saying to yourself, if you can possibly avoid it, 'But how can it possibly be like that?' because you will go down the drain into a blind alley from which nobody has yet escaped. Nobody knows how it can be like that. [Richard Feynman] • Any one who is not shocked by quantum mechanics has not fu ...
CHM 441: QUANTUM CHEMISTRY
... applied. The operators of quantum mechanics are linear. A linear operator has the following properties:- ...
... applied. The operators of quantum mechanics are linear. A linear operator has the following properties:- ...
Announcements
... energy and momentum bring out particle properties, while experiments designed to examine spatial distribution of energy bring out wavelike properties ...
... energy and momentum bring out particle properties, while experiments designed to examine spatial distribution of energy bring out wavelike properties ...
2.8-2.9 - BYU Physics and Astronomy
... parameters. Write a paragraph about how the simulation supports the discussion of the photoelectric effect from the book and any insights you gained from performing the simulation. You may include figures or a table if you'd like. ...
... parameters. Write a paragraph about how the simulation supports the discussion of the photoelectric effect from the book and any insights you gained from performing the simulation. You may include figures or a table if you'd like. ...
Reverse Causality and the Transactional Interpretation
... Interference is still present, even when an unambiguous Welcher-Weg (which-way) experiment is performed. ...
... Interference is still present, even when an unambiguous Welcher-Weg (which-way) experiment is performed. ...
Chapter 7 -- Radiative Corrections: some formal developments Chapter 7:
... the ground state was observed to determine the transition rate. From this, they were able to deduce the shift between the and states.” ...
... the ground state was observed to determine the transition rate. From this, they were able to deduce the shift between the and states.” ...