A modern view of forces - HEP Educational Outreach
... • In the H atom, the electron is bound due to the continual exchange of photons between the electron and the proton. • The strength of that interaction is directly proportional to the EM coupling constant, aem! • In general, all force carriers will “couple to” particles with some “strength”. – The p ...
... • In the H atom, the electron is bound due to the continual exchange of photons between the electron and the proton. • The strength of that interaction is directly proportional to the EM coupling constant, aem! • In general, all force carriers will “couple to” particles with some “strength”. – The p ...
Fall Exam 3 - Chemistry - University of Kentucky
... Which one of the following is not true regarding the wave nature of light? ...
... Which one of the following is not true regarding the wave nature of light? ...
Light, Energy, and More
... • Particle and wave characteristics • Light is a beam of tiny particles, called photons, acting like a wave ...
... • Particle and wave characteristics • Light is a beam of tiny particles, called photons, acting like a wave ...
GPS, Clocks and Relativity dark - ION Southern California Section
... a Lorentz transformation. We can circumvent this difficulty by a frequently used stratagem (elevated by some to the status of an additional postulate of relativity). We imagine an infinity of inertial systems moving uniformly relative to the laboratory system, one of which instantaneously matches th ...
... a Lorentz transformation. We can circumvent this difficulty by a frequently used stratagem (elevated by some to the status of an additional postulate of relativity). We imagine an infinity of inertial systems moving uniformly relative to the laboratory system, one of which instantaneously matches th ...
abstract.
... disk is supposed to rotate synchronously with the (very low frequency!) emitted photons. This disk is conceived so as to be conducting along only one axis in the xy plane (this can be achieved if the disk is composed of metallic thin wires aligned along a single direction within xy). Let us look at ...
... disk is supposed to rotate synchronously with the (very low frequency!) emitted photons. This disk is conceived so as to be conducting along only one axis in the xy plane (this can be achieved if the disk is composed of metallic thin wires aligned along a single direction within xy). Let us look at ...
1 Hot Electron Modeling I: Extended Drift–Diffusion Models
... one wants to use the method for engineering applications, involving intensive computations. Further approximations are possible if the transport properties of a particular material (e.g. Si or GaAs) are well known, or if a special geometry allows one to make assumptions on the electric fields or on ...
... one wants to use the method for engineering applications, involving intensive computations. Further approximations are possible if the transport properties of a particular material (e.g. Si or GaAs) are well known, or if a special geometry allows one to make assumptions on the electric fields or on ...
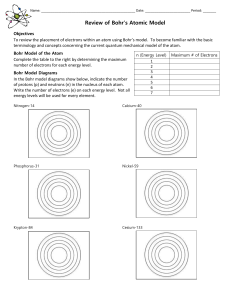
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
IBA Superconducting Synchrocyclotron for Proton Therapy: Central
... they do not undergo continuous acceleration and are lost. “F3" represents particles for which the outward radial swing is too small to compensate the inward swing; these particles return to the machine centre and are lost. ...
... they do not undergo continuous acceleration and are lost. “F3" represents particles for which the outward radial swing is too small to compensate the inward swing; these particles return to the machine centre and are lost. ...
Chapter 3
... –2.04 x 10 J = –hR (1/1 – 1/16) hR = 2.18 x 10–18 J (a) –hR (1/4 – 1/9) = –2.18 x 10–18 J x 0.139 = –3.02 x 10–19 J The energy of the photon emitted is 3.02 x 10–19 J. (4%) (b) The energy of the photon emitted is 3.02 x 10–19 J, because orbitals in the hydrogen atom with the same principle quantum n ...
... –2.04 x 10 J = –hR (1/1 – 1/16) hR = 2.18 x 10–18 J (a) –hR (1/4 – 1/9) = –2.18 x 10–18 J x 0.139 = –3.02 x 10–19 J The energy of the photon emitted is 3.02 x 10–19 J. (4%) (b) The energy of the photon emitted is 3.02 x 10–19 J, because orbitals in the hydrogen atom with the same principle quantum n ...
Exploration of a Method to Image an N 2 Molecular Orbital Using the ATI Spectrum
... this case, called Smatrix theory. This method approximates the total timedependent wave function assuming the knowledge of the initial state and final state (and sometimes even some intermediate state) wave function. Smatrix theory creates a series expansion of the total wave function. Success ...
... this case, called Smatrix theory. This method approximates the total timedependent wave function assuming the knowledge of the initial state and final state (and sometimes even some intermediate state) wave function. Smatrix theory creates a series expansion of the total wave function. Success ...
Chapter 5 Electrons in Atoms - Lakeland Regional High School
... A quantum is the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
... A quantum is the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
Impulse and Momentum
... Before he can move, a tackler, running at a velocity of +4.8 m/s, grabs him. The tackler holds onto the receiver, and the two move off together with a velocity of +2.6 m/s. The mass of the tackler is 116 kg. Assuming that momentum is conserved, find the mass of the receiver. ...
... Before he can move, a tackler, running at a velocity of +4.8 m/s, grabs him. The tackler holds onto the receiver, and the two move off together with a velocity of +2.6 m/s. The mass of the tackler is 116 kg. Assuming that momentum is conserved, find the mass of the receiver. ...