Lesson3
... so cleanly and you have to figure it out: ~ ( x) ~1 ( x) ~2 ( x) ~N ( x), x a, b ~ ( x) ( x) b ~ ( x' ) dx' ...
... so cleanly and you have to figure it out: ~ ( x) ~1 ( x) ~2 ( x) ~N ( x), x a, b ~ ( x) ( x) b ~ ( x' ) dx' ...
x(x)
... so cleanly and you have to figure it out: ~ ( x) ~1 ( x) ~2 ( x) ~N ( x), x a, b ~ ( x) ( x) b ~ ( x' ) dx' ...
... so cleanly and you have to figure it out: ~ ( x) ~1 ( x) ~2 ( x) ~N ( x), x a, b ~ ( x) ( x) b ~ ( x' ) dx' ...
Lecture6.QM.to.Lagrangian.Densities
... Quantization arises from placing boundary conditions on the wave function. It is a mathematical result! ...
... Quantization arises from placing boundary conditions on the wave function. It is a mathematical result! ...
Quantum Theory of Hydrogen
... In fact, our electron wave functions are “stationary states.” That helps us understand why the electrons don’t radiate continuously, although we haven’t yet done the mathematics to show this. Now it’s time to do “that” mathematics. ...
... In fact, our electron wave functions are “stationary states.” That helps us understand why the electrons don’t radiate continuously, although we haven’t yet done the mathematics to show this. Now it’s time to do “that” mathematics. ...
D NAME: 1. What is the eigenvalue of Lz for Ψ if the eigenval
... Which of the following statements is/are false for a given set of QMHO wave functions corresponding to the same harmonic potential V? ...
... Which of the following statements is/are false for a given set of QMHO wave functions corresponding to the same harmonic potential V? ...
The energy eigenvalue is E = p2 2m = ¯h2k2 2m = ¯h2 2m (2π L )2
... This is the Airy Equation, and the solution is the Airy Function Ai(z), plotted in Figure 2.1. The Airy Function has a peculiar behavior, oscillatory for negative values of the argument, and decreasing rapidly towards zero for positive values. Of course, this is exactly the behavior we expect for th ...
... This is the Airy Equation, and the solution is the Airy Function Ai(z), plotted in Figure 2.1. The Airy Function has a peculiar behavior, oscillatory for negative values of the argument, and decreasing rapidly towards zero for positive values. Of course, this is exactly the behavior we expect for th ...
“Nature is made in such a way as to be able to be understood
... What is light really?? Or what is electron really?? A particle? Or a wave? It is like.... They can not exist as both. Sometimes they act as wave and sometimes they act as particles... Both nature cannot be manifest at the same time, yet electron or light Is both at the same time! We have two contrad ...
... What is light really?? Or what is electron really?? A particle? Or a wave? It is like.... They can not exist as both. Sometimes they act as wave and sometimes they act as particles... Both nature cannot be manifest at the same time, yet electron or light Is both at the same time! We have two contrad ...
Erwin Schroedinger, Max Born and Wave Mechanics
... German theoretical physicist who was a great contributor to quantum mechanics- discovery of allotropic forms of hydrogen Was awarded the Nobel Prize in Physics for 1932 "for the creation of quantum mechanics". Studied physics under Max Born and soon became his assistant Most famous for his discovery ...
... German theoretical physicist who was a great contributor to quantum mechanics- discovery of allotropic forms of hydrogen Was awarded the Nobel Prize in Physics for 1932 "for the creation of quantum mechanics". Studied physics under Max Born and soon became his assistant Most famous for his discovery ...
Quantum Tunneling - Santa Rosa Junior College
... definite in their properties, (position, energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle simultaneously. We can never understa ...
... definite in their properties, (position, energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle simultaneously. We can never understa ...
Quantum Mechanics Lecture 1 Dr. Mauro Ferreira
... ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
... ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
M 225 Test 2 A Name__________________ SHOW
... constant, and there are only two possible outcomes (either a fan or not). So it’s a binomial experiment. b. What is the probability that out of the three women exactly two women consider themselves a basketball fan? ...
... constant, and there are only two possible outcomes (either a fan or not). So it’s a binomial experiment. b. What is the probability that out of the three women exactly two women consider themselves a basketball fan? ...
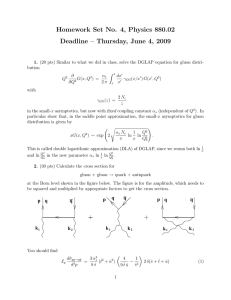
Homework Set No. 4, Physics 880.02
... with the Mandelstam variables ŝ = (k1 + k2 )2 , t̂ = (k1 − p)2 , û = (k2 − p)2 . The factor of 2 in front of the δ-function in Eq. (1) comes from the fact that either the quark or the antiquark can carry momentum p. (q and q̄ in the figure denote the quark and the antiquark. Time flows upward.) A ...
... with the Mandelstam variables ŝ = (k1 + k2 )2 , t̂ = (k1 − p)2 , û = (k2 − p)2 . The factor of 2 in front of the δ-function in Eq. (1) comes from the fact that either the quark or the antiquark can carry momentum p. (q and q̄ in the figure denote the quark and the antiquark. Time flows upward.) A ...
Quantum eraser
... Now, is it possible to ”erase” the which path data from the system and reinstate our interference pattern? Let us examine a simple information erasing scheme by modeling our system with qubits. Say we have a 2 slit apparatus in which we can mark the path the particle takes using qubits A and B each ...
... Now, is it possible to ”erase” the which path data from the system and reinstate our interference pattern? Let us examine a simple information erasing scheme by modeling our system with qubits. Say we have a 2 slit apparatus in which we can mark the path the particle takes using qubits A and B each ...
1_Quantum theory_ introduction and principles
... (operator)(function)=(constant)×(same function) The eigenvalue is the energy E. The set of eigenvalues are the only values that the energy can have (quantization). The eigenfunctions of the Hamiltonian operator H are the wavefunctions of the system. To each eigenvalue corresponds a set of ei ...
... (operator)(function)=(constant)×(same function) The eigenvalue is the energy E. The set of eigenvalues are the only values that the energy can have (quantization). The eigenfunctions of the Hamiltonian operator H are the wavefunctions of the system. To each eigenvalue corresponds a set of ei ...
Solution - UIUC Math
... Diamonds, Hearts (respectively). Then by the Inclusion–Exclusion Principle, P (S ∪ C ∪ D ∪ H) = P (S) + P (C) + P (D) + P (H) − P (SC) − P (SD) − · · · + P (SCD) + P (SCH) + · · · − P (SCDH) µ ¶ ¡39¢ µ ¶ ¡26¢ µ ¶ ¡13¢ ...
... Diamonds, Hearts (respectively). Then by the Inclusion–Exclusion Principle, P (S ∪ C ∪ D ∪ H) = P (S) + P (C) + P (D) + P (H) − P (SC) − P (SD) − · · · + P (SCD) + P (SCH) + · · · − P (SCDH) µ ¶ ¡39¢ µ ¶ ¡26¢ µ ¶ ¡13¢ ...
Algebra I - Fort Thomas Independent Schools
... A student was performing an experiment that compared a new protein food to the old food for goldfish. He found the mean weight gain for the new food to be 12.8 grams with a standard deviation of 3.5 grams. Later he realized that the scale was out of calibration by 1.5 grams (meaning that the scale w ...
... A student was performing an experiment that compared a new protein food to the old food for goldfish. He found the mean weight gain for the new food to be 12.8 grams with a standard deviation of 3.5 grams. Later he realized that the scale was out of calibration by 1.5 grams (meaning that the scale w ...
Homework 3
... 2. Bohr reasoned that e- energy is quantized. Using his formula calculate the energy and the wavelength of a photon emitted by a hydrogen atom if an electron drops from the n = 6 state to the n = 3 state (h = 6.63 10-34 J, RH = 2.18 10-18 J). ...
... 2. Bohr reasoned that e- energy is quantized. Using his formula calculate the energy and the wavelength of a photon emitted by a hydrogen atom if an electron drops from the n = 6 state to the n = 3 state (h = 6.63 10-34 J, RH = 2.18 10-18 J). ...
Probability amplitude

In quantum mechanics, a probability amplitude is a complex number used in describing the behaviour of systems. The modulus squared of this quantity represents a probability or probability density.Probability amplitudes provide a relationship between the wave function (or, more generally, of a quantum state vector) of a system and the results of observations of that system, a link first proposed by Max Born. Interpretation of values of a wave function as the probability amplitude is a pillar of the Copenhagen interpretation of quantum mechanics. In fact, the properties of the space of wave functions were being used to make physical predictions (such as emissions from atoms being at certain discrete energies) before any physical interpretation of a particular function was offered. Born was awarded half of the 1954 Nobel Prize in Physics for this understanding (see #References), and the probability thus calculated is sometimes called the ""Born probability"". These probabilistic concepts, namely the probability density and quantum measurements, were vigorously contested at the time by the original physicists working on the theory, such as Schrödinger and Einstein. It is the source of the mysterious consequences and philosophical difficulties in the interpretations of quantum mechanics—topics that continue to be debated even today.