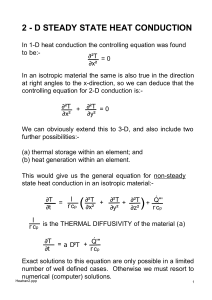
science grade 7 blizzard bag assignment
... the particles of a substance is called thermal energy. Temperature is the average amount of energy of motion in each particle of a substance. It is a measure of how or cold a substance is. Temperature is one of the most important elements of weather. Air temperature is usually measured with a thermo ...
... the particles of a substance is called thermal energy. Temperature is the average amount of energy of motion in each particle of a substance. It is a measure of how or cold a substance is. Temperature is one of the most important elements of weather. Air temperature is usually measured with a thermo ...
Lab 1
... container and measure the temperature of the warm water. If you start with water about 5 oC to 10oC above room temperature and end with the water about 5 oC to 10oC below room temperature, the heat that sneaks into the cooler room from the warm water will nearly cancel the heat that sneaks into the ...
... container and measure the temperature of the warm water. If you start with water about 5 oC to 10oC above room temperature and end with the water about 5 oC to 10oC below room temperature, the heat that sneaks into the cooler room from the warm water will nearly cancel the heat that sneaks into the ...
Chapter Two Atoms & The Periodic Table
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
Joule`s Law and Heat Transfer Name:
... (transformer), calorimeter: jacket and cup, electronic balance, cold-water (<20oC), ice, digital multi-meters (2), and banana-plug wires (5: 2-Red and 3-Black). Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured ...
... (transformer), calorimeter: jacket and cup, electronic balance, cold-water (<20oC), ice, digital multi-meters (2), and banana-plug wires (5: 2-Red and 3-Black). Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured ...
energy
... What is Absolute Zero? • The point at which no more heat can be removed from a system • Almost no movement of molecules • Theoretical temperature ...
... What is Absolute Zero? • The point at which no more heat can be removed from a system • Almost no movement of molecules • Theoretical temperature ...
how to wire electric heat relays - Grover Electric and Plumbing Supply
... therm o sta ts; how ever i t is e xtremely difficult to adjust them so that the temperature throughout the area remains even. Two thermostats in one room tend to burden one heat source with more than its share of the work load. In areas requiring over 5,500 watts of heat, it is almo st always necess ...
... therm o sta ts; how ever i t is e xtremely difficult to adjust them so that the temperature throughout the area remains even. Two thermostats in one room tend to burden one heat source with more than its share of the work load. In areas requiring over 5,500 watts of heat, it is almo st always necess ...
Chapters 19&20
... • Quantity Q + W = ΔEint (change of internal energy) is path-independent • 1st law of thermodynamics: the internal energy of a system increases if heat is added to the system or work is done on the system ...
... • Quantity Q + W = ΔEint (change of internal energy) is path-independent • 1st law of thermodynamics: the internal energy of a system increases if heat is added to the system or work is done on the system ...
Joule`s Law and Heat Transfer Name:
... (transformer), calorimeter: jacket and cup, cold-water (<20oC), ice, balance, digital multimeters (2), and banana-plug wires (5). Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured in calories. In this activity w ...
... (transformer), calorimeter: jacket and cup, cold-water (<20oC), ice, balance, digital multimeters (2), and banana-plug wires (5). Theory: We will use electrical energy to heat a certain amount of cold-water. Electrical energy is measured in Joules and heat is measured in calories. In this activity w ...
Chapter 11 1. While checking the temperature of an IC. chip the
... with an inside temperature of 4500F sitting in a room of temperature 720F. What is the heat flow out of the oven in BTU? 7. A power transistor bank is mounted on a 1kg aluminum heat sink. The transistors dissapates 500W and all this heat flows into the sink. What is the change in temperature of the ...
... with an inside temperature of 4500F sitting in a room of temperature 720F. What is the heat flow out of the oven in BTU? 7. A power transistor bank is mounted on a 1kg aluminum heat sink. The transistors dissapates 500W and all this heat flows into the sink. What is the change in temperature of the ...
Heat - Warren County Schools
... Transfer of heat from one substance to another by direct contact of molecules. Happens in solids, liquids and gases Best conduction happens in solids Examples: Sauce pan on a stove top, metal spoon in a bowl of soup, ice melting in a warm hand, hot shower, walking on hot coals ...
... Transfer of heat from one substance to another by direct contact of molecules. Happens in solids, liquids and gases Best conduction happens in solids Examples: Sauce pan on a stove top, metal spoon in a bowl of soup, ice melting in a warm hand, hot shower, walking on hot coals ...
1-14 The filament of a 150 W incandescent lamp is 5 cm long and
... rate of heat transfer through the wall is to be determined. Assumptions 1 Steady operating conditions exist since the surface temperatures of the wall remain constant at the specified values. 2 Thermal properties of the wall are constant. Properties The thermal conductivity of the wall is given to b ...
... rate of heat transfer through the wall is to be determined. Assumptions 1 Steady operating conditions exist since the surface temperatures of the wall remain constant at the specified values. 2 Thermal properties of the wall are constant. Properties The thermal conductivity of the wall is given to b ...
Cold Weather Heat Pump Operation Air to Air heat Pump Systems
... During the heating mode the unit extracts heat from the outside air and rejects it into the living space. During the cooling mode the unit extracts heat from the inside space and rejects it outside. As you can see from this operation the colder it gets the harder the machine has to work to extract h ...
... During the heating mode the unit extracts heat from the outside air and rejects it into the living space. During the cooling mode the unit extracts heat from the inside space and rejects it outside. As you can see from this operation the colder it gets the harder the machine has to work to extract h ...
Mechanical & Thermal Energy Energy
... The sum of all kinetic energies of all the particles comprising an object is thermal energy. (most matter expands as its thermal energy increases) The faster molecules are moving, the more thermal energy they have; which is why balls go farther in warm weather than cold. ...
... The sum of all kinetic energies of all the particles comprising an object is thermal energy. (most matter expands as its thermal energy increases) The faster molecules are moving, the more thermal energy they have; which is why balls go farther in warm weather than cold. ...
Exam 5 Physics 124A Fall 2003 Name:
... 7. Which of the following statements is true when 60 kg of water initially at 20ºC is completely frozen to become ice at 0ºC? (The latent heat of fusion of water is 33.5×104 J/kg. The specific heat of water is 4186 J/kg/Cº) (A) 8.4×106 J of energy is released (B) 8.4×106 J of energy is absorbed (C) ...
... 7. Which of the following statements is true when 60 kg of water initially at 20ºC is completely frozen to become ice at 0ºC? (The latent heat of fusion of water is 33.5×104 J/kg. The specific heat of water is 4186 J/kg/Cº) (A) 8.4×106 J of energy is released (B) 8.4×106 J of energy is absorbed (C) ...
Rocket! - Teach Engineering
... The energy in the fuel that is not lost to the heat of combustion is converted into work energy to propel the rocket into the air Combustion reactions require fuel, oxygen and heat The energy of combustion comes from the breaking ...
... The energy in the fuel that is not lost to the heat of combustion is converted into work energy to propel the rocket into the air Combustion reactions require fuel, oxygen and heat The energy of combustion comes from the breaking ...
Energy diagrams 4: First law of thermodynamics
... in a system is conserved. For any chemical reaction, the overall change in energy of a system (U) is caused by both the work done to or by the system (w) and the change in temperature (or heat) of the chemicals in the reaction (q). This definition is written as: ...
... in a system is conserved. For any chemical reaction, the overall change in energy of a system (U) is caused by both the work done to or by the system (w) and the change in temperature (or heat) of the chemicals in the reaction (q). This definition is written as: ...
Solid Oxide Fuel Cells For Power Generation
... Solid oxide fuel cell (SOFC) is an electrochemical device, based on an oxide ion conducting electrolyte, which converts chemical energy of a fuel (such as hydrogen or a hydrocarbon) into electricity at temperatures from about 550 to 1000oC. SOFC offers certain advantages over lower temperature fuel ...
... Solid oxide fuel cell (SOFC) is an electrochemical device, based on an oxide ion conducting electrolyte, which converts chemical energy of a fuel (such as hydrogen or a hydrocarbon) into electricity at temperatures from about 550 to 1000oC. SOFC offers certain advantages over lower temperature fuel ...
Specific Heat!
... Why? The sun has been beating down on both of them for the same amount of time........... ...
... Why? The sun has been beating down on both of them for the same amount of time........... ...
Student Notes Page
... – _________________ (electromagnetic waves such as the sun or a microwave oven) Heat is a form of energy associated with the ___________________________ of an object. • Heat is different from temperature; heat is transferred, and cannot be measured directly. Temperature can be measured directly. Tem ...
... – _________________ (electromagnetic waves such as the sun or a microwave oven) Heat is a form of energy associated with the ___________________________ of an object. • Heat is different from temperature; heat is transferred, and cannot be measured directly. Temperature can be measured directly. Tem ...
Cogeneration

Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Trigeneration or combined cooling, heat and power (CCHP) refers to the simultaneous generation of electricity and useful heating and cooling from the combustion of a fuel or a solar heat collector. Cogeneration is a thermodynamically efficient use of fuel. In separate production of electricity, some energy must be discarded as waste heat, but in cogeneration this thermal energy is put to use. All thermal power plants emit heat during electricity generation, which can be released into the natural environment through cooling towers, flue gas, or by other means. In contrast, CHP captures some or all of the by-product for heating, either very close to the plant, or—especially in Scandinavia and Eastern Europe—as hot water for district heating with temperatures ranging from approximately 80 to 130 °C. This is also called combined heat and power district heating (CHPDH). Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling.The supply of high-temperature heat first drives a gas or steam turbine-powered generator and the resulting low-temperature waste heat is then used for water or space heating as described in cogeneration. At smaller scales (typically below 1 MW) a gas engine or diesel engine may be used. Trigeneration differs from cogeneration in that the waste heat is used for both heating and cooling, typically in an absorption refrigerator. CCHP systems can attain higher overall efficiencies than cogeneration or traditional power plants. In the United States, the application of trigeneration in buildings is called building cooling, heating and power (BCHP). Heating and cooling output may operate concurrently or alternately depending on need and system construction.Cogeneration was practiced in some of the earliest installations of electrical generation. Before central stations distributed power, industries generating their own power used exhaust steam for process heating. Large office and apartment buildings, hotels and stores commonly generated their own power and used waste steam for building heat. Due to the high cost of early purchased power, these CHP operations continued for many years after utility electricity became available.