MgO thermo lab
... A foam cup and cover will be used as the calorimeter since it does not absorb very much heat itself and acts a good insulator to prevent transfer of heat between the contents of the calorimeter and the surroundings. Preparing the Calculator and CBL and Collecting Data 1. Attach the CBL temperature p ...
... A foam cup and cover will be used as the calorimeter since it does not absorb very much heat itself and acts a good insulator to prevent transfer of heat between the contents of the calorimeter and the surroundings. Preparing the Calculator and CBL and Collecting Data 1. Attach the CBL temperature p ...
General Physics (PHY 2130) - Wayne State University Physics and
... processes. Must include: • Q ...
... processes. Must include: • Q ...
heat capacity
... b) Heat always flows from warmer objects to cooler objects. c) Adding heat can cause an increase in the temperature of an object. d) Heat cannot be specifically detected by senses or ...
... b) Heat always flows from warmer objects to cooler objects. c) Adding heat can cause an increase in the temperature of an object. d) Heat cannot be specifically detected by senses or ...
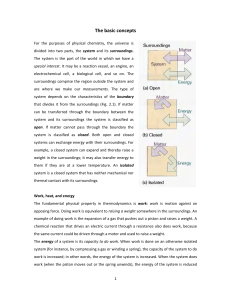
The basic concepts For the purposes of physical chemistry, the
... The concept of temperature springs from the observation that a change in physical state (for example, a change of volume) can occur when two objects are in contact with one another, as when a red-hot metal is plunged into water. Later we shall see that the change in state can be interpreted as arisi ...
... The concept of temperature springs from the observation that a change in physical state (for example, a change of volume) can occur when two objects are in contact with one another, as when a red-hot metal is plunged into water. Later we shall see that the change in state can be interpreted as arisi ...
Electrical conduction
... 2.1 Classical theory : The DRUDE model Goal: To find out the relation between the conductivity (or resistivity) and drift velocity , and thereby its relation to mean free time and drift mobility, from the description of the current density In a conductor where ‘e’ drift in the presence of an elect ...
... 2.1 Classical theory : The DRUDE model Goal: To find out the relation between the conductivity (or resistivity) and drift velocity , and thereby its relation to mean free time and drift mobility, from the description of the current density In a conductor where ‘e’ drift in the presence of an elect ...
smart power generation from waste heat by thermo electric generator
... Fig. Schematic diagram of a thermoelectric generator. ...
... Fig. Schematic diagram of a thermoelectric generator. ...
1 2 mc = 3 2 (R / N )T R / N is the (gas constant) / molecule and is
... Experimental Evidence for Kinetic Theory:Heat Capacities Definition: Heat in calories needed to raise temperature of 1 mole of a substance 1° centigrade. Two kinds: Cp (add heat at constant pressure) Cv (add heat at constant volume) ...
... Experimental Evidence for Kinetic Theory:Heat Capacities Definition: Heat in calories needed to raise temperature of 1 mole of a substance 1° centigrade. Two kinds: Cp (add heat at constant pressure) Cv (add heat at constant volume) ...
Chapter 12
... If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy Degradation of energy is the conversion of high-grade energy to ...
... If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy Degradation of energy is the conversion of high-grade energy to ...
Complete Paper
... thermal capacity of the collector, and the connecting pipes including fluid flow and on the pattern of hot water use. All components were designed for and constructed in line with the design values obtained. The system was tested on a normal sunny day, rainy day, and cloudy day between the hours of ...
... thermal capacity of the collector, and the connecting pipes including fluid flow and on the pattern of hot water use. All components were designed for and constructed in line with the design values obtained. The system was tested on a normal sunny day, rainy day, and cloudy day between the hours of ...
Chapter 4 - UniMAP Portal
... energy required to raise the temperature of the unit mass of a substance by one degree as the volume is maintained constant. Specific heat at constant pressure, cp: The energy required to raise the temperature of the unit mass of a substance by one degree as the pressure is maintained constant. ...
... energy required to raise the temperature of the unit mass of a substance by one degree as the volume is maintained constant. Specific heat at constant pressure, cp: The energy required to raise the temperature of the unit mass of a substance by one degree as the pressure is maintained constant. ...
Document
... kilojoules) transferred when one can (about 350 g) is cooled from 25 °C to 3 °C. q = (specific heat) x (mass of substance) x ∆T ...
... kilojoules) transferred when one can (about 350 g) is cooled from 25 °C to 3 °C. q = (specific heat) x (mass of substance) x ∆T ...
HANDBOOK OF FOOD SCIENCE, TECHNOLOGY - FEA
... resistance is only due to the food composition and structure. Knowing the type and extent of the driving forces involved transport parameters can be calculated (9). The main mechanisms involved in heat transfer in food processing are conduction and convection. Conduction is transmitted within a soli ...
... resistance is only due to the food composition and structure. Knowing the type and extent of the driving forces involved transport parameters can be calculated (9). The main mechanisms involved in heat transfer in food processing are conduction and convection. Conduction is transmitted within a soli ...
chapter 5 lecture notes ppt
... When a 3.88 g sample of solid ammonium nitrate disolves in 60.0 g of water in a coffee cup calorimeter, the temperature drops from 23.0 °C to 18.4 °C. (a) Calculate H (in kJ/mol ammonium nitrate) for the solution process. Assume that the specific heat is constant and = 4.184 J/g°C. (b) Is this proc ...
... When a 3.88 g sample of solid ammonium nitrate disolves in 60.0 g of water in a coffee cup calorimeter, the temperature drops from 23.0 °C to 18.4 °C. (a) Calculate H (in kJ/mol ammonium nitrate) for the solution process. Assume that the specific heat is constant and = 4.184 J/g°C. (b) Is this proc ...
Chapter 15
... The second law of thermodynamics states that all spontaneous processes proceeding in an isolated system lead to an increase in entropy. In other words, an isolated system will naturally pursue a state of higher disorder. If you watch a magician throw a deck of cards into the air, you would expect th ...
... The second law of thermodynamics states that all spontaneous processes proceeding in an isolated system lead to an increase in entropy. In other words, an isolated system will naturally pursue a state of higher disorder. If you watch a magician throw a deck of cards into the air, you would expect th ...
Is there a negative absolute temperature?
... second law. It lacks additivity, essential for the validity of thermodynamics • For classical Hamiltonian systems, SG satisfies an exact adiabatic invariance (due to Hertz) while Boltzmann entropy does not. However, the violations are of order 1/N and go away for large systems • Thermodynamics is a ...
... second law. It lacks additivity, essential for the validity of thermodynamics • For classical Hamiltonian systems, SG satisfies an exact adiabatic invariance (due to Hertz) while Boltzmann entropy does not. However, the violations are of order 1/N and go away for large systems • Thermodynamics is a ...
4 - web page for staff
... neither created nor destroyed, although equal amounts of positive and negative charge may be simultaneously created, obtained by separation, destroyed, or lost by recombination.” The integral form of the continuity equation, ...
... neither created nor destroyed, although equal amounts of positive and negative charge may be simultaneously created, obtained by separation, destroyed, or lost by recombination.” The integral form of the continuity equation, ...