Paul Ehrenfest: The Genesis of the Adiabatic Hypothesis, 1911–1914
... EINSTEIN and STERN.4 Then, in another article of 1913 on a mechanical theorem of BOLTZMANN,5 EHRENFEST already employed a primitive adiabatic hypothesis but did not formulate it clearly. He used it instead to deduce a set of quantization rules that differed from the usual ones and specifically obtai ...
... EINSTEIN and STERN.4 Then, in another article of 1913 on a mechanical theorem of BOLTZMANN,5 EHRENFEST already employed a primitive adiabatic hypothesis but did not formulate it clearly. He used it instead to deduce a set of quantization rules that differed from the usual ones and specifically obtai ...
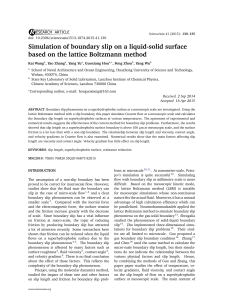
Simulation of boundary slip on a liquid
... different times with LBM simulation. y = h/H where H is the gap between the two plates and h is the distance from fluid point to the bottom plate and the velocity is dimensionless speed in the LBM. ...
... different times with LBM simulation. y = h/H where H is the gap between the two plates and h is the distance from fluid point to the bottom plate and the velocity is dimensionless speed in the LBM. ...
Thermodynamics & Statistical Mechanics:
... substance (e.g., in twelve grams of carbon, or eighteen grams of water) there are ...
... substance (e.g., in twelve grams of carbon, or eighteen grams of water) there are ...
- PhilSci
... box of the previous section replacing the abstract notion of “observable” by a concrete interaction between subsystems. Say we have the N balls in a box as above, but now we add a new set of 2N “small” balls2 , with negligible mass, that do not interact among themselves but interact with the previou ...
... box of the previous section replacing the abstract notion of “observable” by a concrete interaction between subsystems. Say we have the N balls in a box as above, but now we add a new set of 2N “small” balls2 , with negligible mass, that do not interact among themselves but interact with the previou ...
Chapter 3. The Second Law
... W : the number of microstates : the ways in which the molecules of a system can be arranged while keeping the total energy constant When W = 1, S = 0 When molecules can access more microstates for a given energy (e.g. as the system volume increases), the entropy increases Molecules in a system at hi ...
... W : the number of microstates : the ways in which the molecules of a system can be arranged while keeping the total energy constant When W = 1, S = 0 When molecules can access more microstates for a given energy (e.g. as the system volume increases), the entropy increases Molecules in a system at hi ...
Beginning Research on the Quantification of Spatial Order
... through a succession of configurations, but rather to determine how an ensemble of systems will evolve, with respect to their detailed configurations (Gibbs 1902). In this connection, a macrostate is a configuration of a system specified by certain parameters like temperature, pressure, and volume, ...
... through a succession of configurations, but rather to determine how an ensemble of systems will evolve, with respect to their detailed configurations (Gibbs 1902). In this connection, a macrostate is a configuration of a system specified by certain parameters like temperature, pressure, and volume, ...
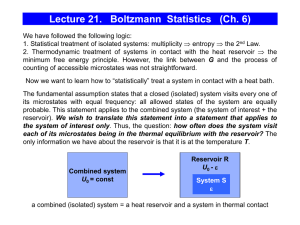
Lecture 21. Boltzmann Statistics (Ch. 6)
... they still might participate in the so-called exchange interaction which is dependent on the spin of interacting particles (this is related to the principle of indistinguishability of elementary particles, we’ll consider bosons and fermions in Lecture 23). This type of interactions becomes important ...
... they still might participate in the so-called exchange interaction which is dependent on the spin of interacting particles (this is related to the principle of indistinguishability of elementary particles, we’ll consider bosons and fermions in Lecture 23). This type of interactions becomes important ...
Chapter 19 Chemical Thermodynamics
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
Chapter 19 Chemical Thermodynamics
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
Intermediate Physical Chemistry (CHEM2503)
... Two friends A and B are drinking beer in a pub one night. Out of boredom, the two start to play “fifteen-twenty”. A mutual friend C steps in, and the three play together. Of course, this time one hand is used by each. A is quite smart, and studies chemistry in HKU. He figures out a winning strategy. ...
... Two friends A and B are drinking beer in a pub one night. Out of boredom, the two start to play “fifteen-twenty”. A mutual friend C steps in, and the three play together. Of course, this time one hand is used by each. A is quite smart, and studies chemistry in HKU. He figures out a winning strategy. ...
Transport and Non-Equilibrium Dynamics in Optical Lattices
... complicated quantum systems [1]. Since Feynman promoted his idea in the 1980ies, the construction of such a quantum computer is still not within reach. However, physicists are currently exploring different options for a physical realization, and ideas range from quantum dots and novel topological ma ...
... complicated quantum systems [1]. Since Feynman promoted his idea in the 1980ies, the construction of such a quantum computer is still not within reach. However, physicists are currently exploring different options for a physical realization, and ideas range from quantum dots and novel topological ma ...
Chapter 19 Chemical Thermodynamics
... • Entropy (S) is a term coined by Rudolph Clausius in the 19th century. • Clausius was convinced of the significance of the ratio of heat delivered and the temperature at which it is delivered, q T ...
... • Entropy (S) is a term coined by Rudolph Clausius in the 19th century. • Clausius was convinced of the significance of the ratio of heat delivered and the temperature at which it is delivered, q T ...
Chapter 19 Chemical Thermodynamics
... • Molecules exhibit several types of motion: Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or ...
... • Molecules exhibit several types of motion: Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or ...
Entropy Systems Theory
... decreases. But if all available energy has been expended, then no more work can be done, and entropy will either remain constant or increase. 2.2 Boltzmann’s Entropy ...
... decreases. But if all available energy has been expended, then no more work can be done, and entropy will either remain constant or increase. 2.2 Boltzmann’s Entropy ...
S - BEHS Science
... Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or rotation about bonds. ...
... Translational: Movement of the entire molecule from one place to another. Vibrational: Periodic motion of atoms within a molecule. Rotational: Rotation of the molecule on about an axis or rotation about bonds. ...
Introduction to the immersed boundary method
... There is another possibility: The forcing term f in eq. (1) may be used to mimic a boundary condition. A local force density is introduced to modify the flow field. When applied correctly, this can be used to realize deformable or rigid objects in the flow. The advantage of this approach is that com ...
... There is another possibility: The forcing term f in eq. (1) may be used to mimic a boundary condition. A local force density is introduced to modify the flow field. When applied correctly, this can be used to realize deformable or rigid objects in the flow. The advantage of this approach is that com ...
Statistical Thermodynamics and Stochastic The
... Poincare and Lyapunov. John William Rayleigh (1842-1919) is the founder of the theory of nonlinear oscillations. Many applications in optics, acoustics, mechanics and hydrodynamics are connected with his name. Alexander M. Lyapunov was a Russian mathematician, who formulated in 1982 the mathematical ...
... Poincare and Lyapunov. John William Rayleigh (1842-1919) is the founder of the theory of nonlinear oscillations. Many applications in optics, acoustics, mechanics and hydrodynamics are connected with his name. Alexander M. Lyapunov was a Russian mathematician, who formulated in 1982 the mathematical ...
Entropy, a statistical approach
... • reactions in solution or in the same phase which produce more product molecules than reactant molecules tend to have positive entropy changes (a matter of stoichiometry) • dissolution of a solid into solution is normally associated with a positive entropy change, but there are exceptions. ...
... • reactions in solution or in the same phase which produce more product molecules than reactant molecules tend to have positive entropy changes (a matter of stoichiometry) • dissolution of a solid into solution is normally associated with a positive entropy change, but there are exceptions. ...
Entropy: Measure of Diversity?
... As shown in the rightmost term of this formula, every triangular number is a binomial coefficient: the nth triangular is the number of distinct pairs to be selected from n + 1 objects. In this form it solves the 'handshake problem' of counting the number of handshakes if each person in a room shakes ...
... As shown in the rightmost term of this formula, every triangular number is a binomial coefficient: the nth triangular is the number of distinct pairs to be selected from n + 1 objects. In this form it solves the 'handshake problem' of counting the number of handshakes if each person in a room shakes ...
The Physics of Negative Absolute Temperatures
... The concept of negative absolute temperature, first mooted by Onsager[1, 2] in the context of twodimensional turbulence, fits consistently into the description of various experimental findings. In 1951 Purcell and Pound[3], by rapidly reversing the applied magnetic field applied to the nuclear spins ...
... The concept of negative absolute temperature, first mooted by Onsager[1, 2] in the context of twodimensional turbulence, fits consistently into the description of various experimental findings. In 1951 Purcell and Pound[3], by rapidly reversing the applied magnetic field applied to the nuclear spins ...
Definite Integral and the Gibbs Paradox
... Many textbooks [2-4] classify thermodynamic quantities as two classes: extensive or intensive. The following is a typical definition [3]: The quantities we use to describe the macroscopic behavior of a system in equilibrium are called properties, the observable characteristics of a system. Other nam ...
... Many textbooks [2-4] classify thermodynamic quantities as two classes: extensive or intensive. The following is a typical definition [3]: The quantities we use to describe the macroscopic behavior of a system in equilibrium are called properties, the observable characteristics of a system. Other nam ...
Statistical Mechanics--
... d in a volume V , we have L = Nd 2 , where the constant k is of order-of-magnitude 1. While an ad hoc assumption, Maxwell (1831-79), in the first of three great papers on the subject, showed that it was a logical mechanism to explain viscosity, and incorporated it naturally into his theory. Clausius ...
... d in a volume V , we have L = Nd 2 , where the constant k is of order-of-magnitude 1. While an ad hoc assumption, Maxwell (1831-79), in the first of three great papers on the subject, showed that it was a logical mechanism to explain viscosity, and incorporated it naturally into his theory. Clausius ...
Entropy
... Depending on the topic and the context in which it is being used, the term entropy has been used to describe any of numerous phenomena. The word entropy was introduced in 1865 by Rudolf Clausius, a German physicist. Two main areas, thermodynamic entropy (including statistical mechanics) and informat ...
... Depending on the topic and the context in which it is being used, the term entropy has been used to describe any of numerous phenomena. The word entropy was introduced in 1865 by Rudolf Clausius, a German physicist. Two main areas, thermodynamic entropy (including statistical mechanics) and informat ...
2. Classical Gases
... “It has always been believed that Gibbs’s paradox embodied profound thought. That it was intimately linked up with something so important and entirely new could hardly have been foreseen.” Erwin Schrödinger We said earlier that the formula for the partition function (2.7) isn’t quite right. What di ...
... “It has always been believed that Gibbs’s paradox embodied profound thought. That it was intimately linked up with something so important and entirely new could hardly have been foreseen.” Erwin Schrödinger We said earlier that the formula for the partition function (2.7) isn’t quite right. What di ...
Ludwig Boltzmann

Ludwig Eduard Boltzmann (February 20, 1844 – September 5, 1906) was an Austrian physicist and philosopher whose greatest achievement was in the development of statistical mechanics, which explains and predicts how the properties of atoms (such as mass, charge, and structure) determine the physical properties of matter (such as viscosity, thermal conductivity, and diffusion).