Laboratory Works and Home Tasks in General Chemistry
... is described perfectly by its own chemical formula; it is stable at storage, easily dissolved in water, it has a big molar mass (the higher the molar mass of the given initial substance, the smaller the mistake at weighing). Only several substances can entirely or partially satisfy these requirement ...
... is described perfectly by its own chemical formula; it is stable at storage, easily dissolved in water, it has a big molar mass (the higher the molar mass of the given initial substance, the smaller the mistake at weighing). Only several substances can entirely or partially satisfy these requirement ...
Lab Manual (Eng. Medium)
... top (zero) calibration mark and then drained into a separate container until the calibration mark for the desired volume is reached. The remaining liquid is either discarded or returned to its original container. The maximum indicated capacity of some graduated pipettes is delivered by draining to a ...
... top (zero) calibration mark and then drained into a separate container until the calibration mark for the desired volume is reached. The remaining liquid is either discarded or returned to its original container. The maximum indicated capacity of some graduated pipettes is delivered by draining to a ...
МЕТОДИЧЕСКИЕ УКАЗАНИЯ СТУДЕНТАМ
... • Wash off the concentrated acids and alkalies with a strong stream of the running water. Spilled concentrated acids and alcalies mist be cavered with sand and cleaned away. • If a thermometre is broken up and mercury spills, it should be collected with a special trap or a bulb. The minute particle ...
... • Wash off the concentrated acids and alkalies with a strong stream of the running water. Spilled concentrated acids and alcalies mist be cavered with sand and cleaned away. • If a thermometre is broken up and mercury spills, it should be collected with a special trap or a bulb. The minute particle ...
engineering chemistry
... Chemistry is the branch of science that deals with the study of matter, its composition, physical and chemical properties and applications. It is important for engineers to have knowledge of chemistry as those may face problems in fields as diverse as design and development of new materials, quality ...
... Chemistry is the branch of science that deals with the study of matter, its composition, physical and chemical properties and applications. It is important for engineers to have knowledge of chemistry as those may face problems in fields as diverse as design and development of new materials, quality ...
44. Find рН of formic acid solution with mass percent ω=5
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
György Dombi Gerda Szakonyi Authors
... Visual observation of the end-point by using an acidbase indicator is simple and convenient, but it may cause several problems. Instrumental methods are being used in most of the quantitative analyses in the Pharmacopeia; for acidbase titrations, the measurement of pH can be a possible solution. P ...
... Visual observation of the end-point by using an acidbase indicator is simple and convenient, but it may cause several problems. Instrumental methods are being used in most of the quantitative analyses in the Pharmacopeia; for acidbase titrations, the measurement of pH can be a possible solution. P ...
"Cyano Compounds, Inorganic," in: Ullmann`s Encyclopedia of
... residual gases, H2, CO, and N2, can be used for heating or methanated in a separate unit and recycled as feedstock for HCN manufacture [61]. The advantages of the Andrussow process include (1) long catalyst life, up to 10 000 h; (2) well-tested technology, with a simple and safe reaction system; and ...
... residual gases, H2, CO, and N2, can be used for heating or methanated in a separate unit and recycled as feedstock for HCN manufacture [61]. The advantages of the Andrussow process include (1) long catalyst life, up to 10 000 h; (2) well-tested technology, with a simple and safe reaction system; and ...
www.iitvidya.com salt analysis assignment 1. A compound on
... 37. Identify the following : Na2CO3 SO (D) Also mention the oxidation state of S in all the compounds : 38. How is boron obtained from borax ? Give chemical equations with reaction conditions ? Write the structure of B2H6 and its reaction with HCl. 39. A metallic chloride (A) does not respond chrom ...
... 37. Identify the following : Na2CO3 SO (D) Also mention the oxidation state of S in all the compounds : 38. How is boron obtained from borax ? Give chemical equations with reaction conditions ? Write the structure of B2H6 and its reaction with HCl. 39. A metallic chloride (A) does not respond chrom ...
Chemistry Club Demos - 10-8-15
... room temperature for at least 20 minutes. Do not store prepared bottle rockets for more than 24 hours, and keep them out of direct sunlight and away from heat or ignition sources. 4.) Before the demo, quickly loosen the cap and allow any excess methanol to drain out, and then retighten the cap. ...
... room temperature for at least 20 minutes. Do not store prepared bottle rockets for more than 24 hours, and keep them out of direct sunlight and away from heat or ignition sources. 4.) Before the demo, quickly loosen the cap and allow any excess methanol to drain out, and then retighten the cap. ...
CHEMISTRY CET
... An incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is (1) Competing reaction for an SN2 reaction is rearrangement. (2) A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction. (3) A strong nucleophile in an aprotic solvent increases the rate or ...
... An incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is (1) Competing reaction for an SN2 reaction is rearrangement. (2) A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction. (3) A strong nucleophile in an aprotic solvent increases the rate or ...
stoichiometry - einstein classes
... Oxidizing Agents or Reducing Agents : ‘n’ factor = change in oxidation number Or number of electron lost or gained from one mole of the compound. ...
... Oxidizing Agents or Reducing Agents : ‘n’ factor = change in oxidation number Or number of electron lost or gained from one mole of the compound. ...
Discussion Questions
... d. BaCrO4(s) 43. Separate samples of a solution of an unknown soluble ionic compound are treated with KCl, Na2SO4, and NaOH. A precipitate forms only when Na2SO4 is added. Which cations could be present in the unknown soluble ionic compound? 44. What volume of 0.100 M Na3PO4 is required to pre ...
... d. BaCrO4(s) 43. Separate samples of a solution of an unknown soluble ionic compound are treated with KCl, Na2SO4, and NaOH. A precipitate forms only when Na2SO4 is added. Which cations could be present in the unknown soluble ionic compound? 44. What volume of 0.100 M Na3PO4 is required to pre ...
Lecture 25 Notes
... concentration -- the amount of solute dissolved in a given quantity of solvent or solution ...
... concentration -- the amount of solute dissolved in a given quantity of solvent or solution ...
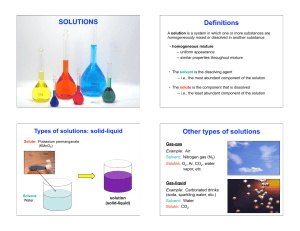
Solutions - iBioKaare
... An ozeotropic mixture of two liquids boils at a lower temperature than either of them when (a) it is saturated (b) it shows positive deviation from Raoult’s law (c) it shows negative deviation from Raoult’s law (d) it is metastable In azeotropic mixture showing positive deviation from Raoult’s law, ...
... An ozeotropic mixture of two liquids boils at a lower temperature than either of them when (a) it is saturated (b) it shows positive deviation from Raoult’s law (c) it shows negative deviation from Raoult’s law (d) it is metastable In azeotropic mixture showing positive deviation from Raoult’s law, ...
1aUnit Two Handouts - Dunmore High School
... Is it a strong base: NaOH, LiOH, KOH, Ba(OH)2, or Ca(OH)2? If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this ...
... Is it a strong base: NaOH, LiOH, KOH, Ba(OH)2, or Ca(OH)2? If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this ...
Chem12 Buffer/Titration : Probs
... 16) If a person breathes too deeply and too rapidly, hyperventilation may occur, in which the pH of the blood becomes too high. A cure for this condition is to breathe several times into a paper bag held tightly over the mouth and nose. In this situation, which one of the following buffer reactions ...
... 16) If a person breathes too deeply and too rapidly, hyperventilation may occur, in which the pH of the blood becomes too high. A cure for this condition is to breathe several times into a paper bag held tightly over the mouth and nose. In this situation, which one of the following buffer reactions ...
2014_S4_CHM_NORMAL (ALL)
... each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: A. B. C. D. ...
... each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the following table: A. B. C. D. ...
The Chemistry of Solutions Page | 1 Unit 7: The Chemistry of
... What is the percent by mass of a solution in which 60.0g of NaOH are dissolved in sufficient water to make 100g of solution? ...
... What is the percent by mass of a solution in which 60.0g of NaOH are dissolved in sufficient water to make 100g of solution? ...
Chemistry of METALS
... mark What would be the importance of heating the ore first before refining it?1 mark To remove the water of crystallization The refined ore has to be dissolved in cryolite first before electrolysis. Why is this necessary? 1½ mark To lower the melting point of aluminium oxide from about 2015oC to 900 ...
... mark What would be the importance of heating the ore first before refining it?1 mark To remove the water of crystallization The refined ore has to be dissolved in cryolite first before electrolysis. Why is this necessary? 1½ mark To lower the melting point of aluminium oxide from about 2015oC to 900 ...
Qualitative Analysis of Anions
... In this lab, you will identify anions in an unknown. Unlike the last lab (Group I Cations), however, you will not be just be using a flow chart in which you separate ions away from each other. Instead, in this lab, you first will perform some preliminary tests using AgNO3, BaCl2, and H2SO4. In these ...
... In this lab, you will identify anions in an unknown. Unlike the last lab (Group I Cations), however, you will not be just be using a flow chart in which you separate ions away from each other. Instead, in this lab, you first will perform some preliminary tests using AgNO3, BaCl2, and H2SO4. In these ...
UNIVERSITI MALAYSIA SABAH
... Weigh out accurately using an analytical balance about 0.2 g of the complex in a weighing bottle. Note the mass of the complex up to the fourth decimal place. Carefully transfer the solid complex into a 250 mL conical flask. Add about 5 mL of 2M H2SO4 to the weighing bottle using a small measuring c ...
... Weigh out accurately using an analytical balance about 0.2 g of the complex in a weighing bottle. Note the mass of the complex up to the fourth decimal place. Carefully transfer the solid complex into a 250 mL conical flask. Add about 5 mL of 2M H2SO4 to the weighing bottle using a small measuring c ...
Chapter 1: Aqueous Processing Systems
... One advantage of the established situation is that the student is exposed to aqueous processing in context, i.e., there can be immediate connection with the student's chosen field. Unfortunately, however, this approach to technical education is not without some disadvantages. First of all, many aqu ...
... One advantage of the established situation is that the student is exposed to aqueous processing in context, i.e., there can be immediate connection with the student's chosen field. Unfortunately, however, this approach to technical education is not without some disadvantages. First of all, many aqu ...
CHM 1033 Chemistry for Health Sciences
... 17. Determine the mass (in grams) of 1.0 liter alcohol, if the density is 0.79 g/ml. What is the specific gravity of the alcohol? 18. An engine part weights 0.82 lb. When measured in a graduated cylinder containing water it displaces a volume of 125.5 ml of water. What is the density of this materia ...
... 17. Determine the mass (in grams) of 1.0 liter alcohol, if the density is 0.79 g/ml. What is the specific gravity of the alcohol? 18. An engine part weights 0.82 lb. When measured in a graduated cylinder containing water it displaces a volume of 125.5 ml of water. What is the density of this materia ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... EXTRA HOMEWORK 4G 1. A stock solution of nitric acid has a concentration of 12.0 M. (a) To what volume should you dilute 50.0 mL of the stock nitric acid solution to obtain a 1.00 M nitric acid solution? (b) If 350. mL of the 1.00 M nitric acid solution is diluted to 4.500 L, what will be the molar ...
... EXTRA HOMEWORK 4G 1. A stock solution of nitric acid has a concentration of 12.0 M. (a) To what volume should you dilute 50.0 mL of the stock nitric acid solution to obtain a 1.00 M nitric acid solution? (b) If 350. mL of the 1.00 M nitric acid solution is diluted to 4.500 L, what will be the molar ...
Heap leaching

Heap leaching is an industrial mining process to extract precious metals, copper, uranium, and other compounds from ore via a series of chemical reactions that absorb specific minerals and then re-separates them after their division from other earth materials. Similar to in situ mining, heap leach mining differs in that it places ore on a liner, then adds the chemicals via drip systems to the ore, whereas in situ mining lacks these liners and pulls pregnant solution up to obtain the minerals. The process has ancient origins; one of the classical methods for the manufacture of copperas (iron sulfate) was to heap up iron pyrite and collect the leachate from the heap, which was then boiled with iron to produce iron sulfate