Gene Technology Regulation 2002
... persons that an institutional biosafety committee has included in the record of assessment as having the appropriate training and experience to undertake the dealing. Similarly, a person complies with paragraph (f) if the facility in which the person undertakes the dealing is in a class ...
... persons that an institutional biosafety committee has included in the record of assessment as having the appropriate training and experience to undertake the dealing. Similarly, a person complies with paragraph (f) if the facility in which the person undertakes the dealing is in a class ...
Safety Administration Implementation Regulation on Agricultural
... III on the basis of the extent of safety increase, with the same classification standard as that of the recipient organisms. 2.The genetic engineered organism obtained from recipient organism of Safety Class III via Type 2 genetic manipulations belongs to Safety Class III 3.The genetic engineered or ...
... III on the basis of the extent of safety increase, with the same classification standard as that of the recipient organisms. 2.The genetic engineered organism obtained from recipient organism of Safety Class III via Type 2 genetic manipulations belongs to Safety Class III 3.The genetic engineered or ...
2.08-11.2 Line Probe Assays
... manufactured under ISO 13485:2003 certification, offering the advantage of quality-assured reagents and test kits, and labeled for use under defined conditions. The tests are also approved by the Regulatory Authority in Europe (CE-Marked) and elsewhere. In-house line probe assays, developed in acade ...
... manufactured under ISO 13485:2003 certification, offering the advantage of quality-assured reagents and test kits, and labeled for use under defined conditions. The tests are also approved by the Regulatory Authority in Europe (CE-Marked) and elsewhere. In-house line probe assays, developed in acade ...
Form GMMO - University of Edinburgh
... (1) It is the responsibility of the Principal Investigator (PI) to undertake a risk assessment in relation to any genetic modification work they, or members of their research group, undertake. The risk assessment must be undertaken and be reviewed and approved by the local GM Safety Committee in adv ...
... (1) It is the responsibility of the Principal Investigator (PI) to undertake a risk assessment in relation to any genetic modification work they, or members of their research group, undertake. The risk assessment must be undertaken and be reviewed and approved by the local GM Safety Committee in adv ...
Synthetic Biology and the CBD
... sample across borders. others, and key genetic diversity from removed and both items retained in Instead, the loot can be developing countries can be identified COP's final decision. They together stored on a memory and recreated using gene editing – all reflect a "two-step process" to address card. ...
... sample across borders. others, and key genetic diversity from removed and both items retained in Instead, the loot can be developing countries can be identified COP's final decision. They together stored on a memory and recreated using gene editing – all reflect a "two-step process" to address card. ...
Ancient DNA Laboratory Guidelines
... these rooms; neither should you, unless you are actively processing aDNA. No Contemporary DNA Allowed The aDNA Laboratory was constructed for the processing of aDNA only. Under no circumstances should fresh tissues or DNA ever enter the premises. aDNA Laboratory Consumables Consumables should never ...
... these rooms; neither should you, unless you are actively processing aDNA. No Contemporary DNA Allowed The aDNA Laboratory was constructed for the processing of aDNA only. Under no circumstances should fresh tissues or DNA ever enter the premises. aDNA Laboratory Consumables Consumables should never ...
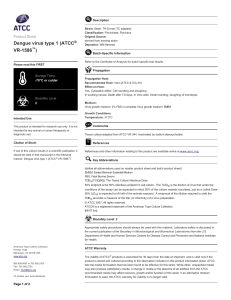
Dengue virus type 1 (ATCC® VR1586™)
... 50% endpoint is the 50% infectious endpoint in cell culture. The TCID50 is the dilution of virus that under the conditions of the assay can be expected to infect 50% of the culture vessels inoculated, just as a Lethal Dose 50% (LD50) is expected to kill half of the animals exposed. A reciprocal of ...
... 50% endpoint is the 50% infectious endpoint in cell culture. The TCID50 is the dilution of virus that under the conditions of the assay can be expected to infect 50% of the culture vessels inoculated, just as a Lethal Dose 50% (LD50) is expected to kill half of the animals exposed. A reciprocal of ...
GM plant viruses - University of Glasgow
... Principal investigator As the principal investigator for this GM project you have a legal responsibility to ensure that all those involved or working on the project have an appropriate level of training and expertise to enable safe working. This includes ensuring that they read and understand this r ...
... Principal investigator As the principal investigator for this GM project you have a legal responsibility to ensure that all those involved or working on the project have an appropriate level of training and expertise to enable safe working. This includes ensuring that they read and understand this r ...
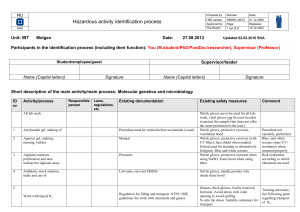
Hazardous activity identification process
... Participants in the identification process (including their function): You (M.student/PhD/PostDoc/researcher), Supervisor (Professor) ...
... Participants in the identification process (including their function): You (M.student/PhD/PostDoc/researcher), Supervisor (Professor) ...
Magnusiomyces capitatus (de Hoog et al.) de Hoog et Smith
... Biosafety Level: 1 Appropriate safety procedures should always be used with this material. Laboratory safety is discussed in the current publication of the Biosafety in Microbiological and Biomedical Laboratories from the U.S. Department of Health and Human Services Centers for Disease Control and P ...
... Biosafety Level: 1 Appropriate safety procedures should always be used with this material. Laboratory safety is discussed in the current publication of the Biosafety in Microbiological and Biomedical Laboratories from the U.S. Department of Health and Human Services Centers for Disease Control and P ...
co-existence more politics than science
... only breeding based on recombinant DNA generates risk to biosafety; all others are safe and need no regulation. ...
... only breeding based on recombinant DNA generates risk to biosafety; all others are safe and need no regulation. ...
Terms of Reference Institutional Biosafety Committee
... To undertake assessment and review of research proposals, to identify any potential biohazard which may arise in the course of research as a consequence of any type of experiment or manipulation which: 1.1.1. May result in the liberation or creation of novel types of nucleic acid with the capacity t ...
... To undertake assessment and review of research proposals, to identify any potential biohazard which may arise in the course of research as a consequence of any type of experiment or manipulation which: 1.1.1. May result in the liberation or creation of novel types of nucleic acid with the capacity t ...
Institution Biosafety Committee (IBC)
... Any personal information is safeguarded by the Privacy Act 1988. This prevents the submitted personal information from being used for purposes other than assessing the certification application, or other circumstances specified by the Gene Technology Act 2000 (Commonwealth). In certain circumstances ...
... Any personal information is safeguarded by the Privacy Act 1988. This prevents the submitted personal information from being used for purposes other than assessing the certification application, or other circumstances specified by the Gene Technology Act 2000 (Commonwealth). In certain circumstances ...
The committee noted that pursuant to the !27th GEAC meeting, M/s
... the DBT was sent to the University of Delhi, with a direction that the DOSSIER be revised as per the comments/suggestions for further review by the Subcommittee and to prepare the risk assessment and risk management document for considerationof the GEAC. The Committee noted that the applicant has re ...
... the DBT was sent to the University of Delhi, with a direction that the DOSSIER be revised as per the comments/suggestions for further review by the Subcommittee and to prepare the risk assessment and risk management document for considerationof the GEAC. The Committee noted that the applicant has re ...
Diamond GM Risk Assessment Guidance
... the possible consequences of an accidental release of a GMM from containment into the environment (including waste disposal, equipment failure and human spread). Consider the local as well as the wider environment. GMMs with the potential to infect or colonise animals and plants are of primary conce ...
... the possible consequences of an accidental release of a GMM from containment into the environment (including waste disposal, equipment failure and human spread). Consider the local as well as the wider environment. GMMs with the potential to infect or colonise animals and plants are of primary conce ...
CH 109, Intro to Chemistry Lab Portland State University Eric
... Lab Manual: Lab Manual for Chemistry 109 available in the Bookstore Materials: You will need chemical splash safety goggles. These are available from the stockroom ($6.50) or the bookstore. Grading: The laboratory is graded on a Pass/No Pass basis. An average of 75% of all points available in the la ...
... Lab Manual: Lab Manual for Chemistry 109 available in the Bookstore Materials: You will need chemical splash safety goggles. These are available from the stockroom ($6.50) or the bookstore. Grading: The laboratory is graded on a Pass/No Pass basis. An average of 75% of all points available in the la ...
Lecture 1 09-23-2016
... Sarchasm! Please don’t take it personally! Set up lunch/coffee meetings! ...
... Sarchasm! Please don’t take it personally! Set up lunch/coffee meetings! ...
pRSI17 Linearized shRNA Cloning and Expression Vector
... Despite the above safety features, use of HIV-based vectors falls within NIH Biosafety Level 2 criteria due to the potential biohazard risk of possible recombination with endogenous viral sequences to form self-replicating virus or the possibility of insertional mutagenesis. For a description of lab ...
... Despite the above safety features, use of HIV-based vectors falls within NIH Biosafety Level 2 criteria due to the potential biohazard risk of possible recombination with endogenous viral sequences to form self-replicating virus or the possibility of insertional mutagenesis. For a description of lab ...
Application for Exemption Status
... Any personal information is safeguarded by the Privacy Act 1988. This prevents the submitted personal information from being used for purposes other than assessing the certification application, or other circumstances specified by the Gene Technology Act 2000 (Commonwealth). In certain circumstances ...
... Any personal information is safeguarded by the Privacy Act 1988. This prevents the submitted personal information from being used for purposes other than assessing the certification application, or other circumstances specified by the Gene Technology Act 2000 (Commonwealth). In certain circumstances ...
Biosafety Protocol Registration Form
... Note: For definition of BSL classifications, refer to Biosafety in Microbiological Biomedical Laboratories (BMBL), or the NIH Guidelines for the use of recombinant DNA. *If the organism is non-pathogenic to humans, check the “No” box. ...
... Note: For definition of BSL classifications, refer to Biosafety in Microbiological Biomedical Laboratories (BMBL), or the NIH Guidelines for the use of recombinant DNA. *If the organism is non-pathogenic to humans, check the “No” box. ...
NIH Guidelines - Institutional Biosafety Committee
... for review and approval or disapproval. • Maintain active communication with the IBC throughout the conduct of the research. Prior to beginning the research (after IBC approval), the PI shall • make all protocols and lab manuals available to all personnel, describing the potential biohazards of th ...
... for review and approval or disapproval. • Maintain active communication with the IBC throughout the conduct of the research. Prior to beginning the research (after IBC approval), the PI shall • make all protocols and lab manuals available to all personnel, describing the potential biohazards of th ...
Preparation and transformation of competent bacteria: Calcium
... techniques, such as PCR, downstream. Keeping all solutions sterile means you can make larger batches of solutions to use over longer periods of time. Otherwise, you will find yourself preparing fresh solutions more often then you would like. Additionally, before disposal we are required to decontami ...
... techniques, such as PCR, downstream. Keeping all solutions sterile means you can make larger batches of solutions to use over longer periods of time. Otherwise, you will find yourself preparing fresh solutions more often then you would like. Additionally, before disposal we are required to decontami ...
GMM Risk Assessment - Queen`s University Belfast
... The containment measures outlined in Part II of Schedule 8 of the Contained Use Regulations will include some measures required where, and to the extent that the risk assessment shows they are required. However additional measures may be in any of the following circumstances: (i) To take full accoun ...
... The containment measures outlined in Part II of Schedule 8 of the Contained Use Regulations will include some measures required where, and to the extent that the risk assessment shows they are required. However additional measures may be in any of the following circumstances: (i) To take full accoun ...
Centrifuge Safety - Department of Chemistry and Biochemistry
... voltage with the use of aqueous solutions – Take precautions to avoid electrocution. – Modern gel boxes have electrodes positioned on the lids to drastically reduce the risk of electrocution. – Always secure the gel box lid before turning on the voltage. Turn off the voltage before removing the lid ...
... voltage with the use of aqueous solutions – Take precautions to avoid electrocution. – Modern gel boxes have electrodes positioned on the lids to drastically reduce the risk of electrocution. – Always secure the gel box lid before turning on the voltage. Turn off the voltage before removing the lid ...
Biochemistry Lab Safety - Department of Chemistry and Biochemistry
... voltage with the use of aqueous solutions – Take precautions to avoid electrocution. – Modern gel boxes have electrodes positioned on the lids to drastically reduce the risk of electrocution. – Always secure the gel box lid before turning on the voltage. Turn off the voltage before removing the lid ...
... voltage with the use of aqueous solutions – Take precautions to avoid electrocution. – Modern gel boxes have electrodes positioned on the lids to drastically reduce the risk of electrocution. – Always secure the gel box lid before turning on the voltage. Turn off the voltage before removing the lid ...