The Quantum Numbers
... The fourth quantum number (ms) is the spin quantum number and describes the electron spin. If two negatively charged particles occupy the same orbital, how do they keep from repelling one another? It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic field ...
... The fourth quantum number (ms) is the spin quantum number and describes the electron spin. If two negatively charged particles occupy the same orbital, how do they keep from repelling one another? It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic field ...
Section 2 Notes
... Returning now to the problem of the atom, it was realized that if, for a moment, we pictured the electron not as a particle but as a wave, then it was possible to get stable configurations. Imagine trying to establish a wave in a circular path about a nucleus. One possibility might look like the ill ...
... Returning now to the problem of the atom, it was realized that if, for a moment, we pictured the electron not as a particle but as a wave, then it was possible to get stable configurations. Imagine trying to establish a wave in a circular path about a nucleus. One possibility might look like the ill ...
Area Courses Electromagnetics, Optics, Photonics
... Please check the University Catalogue for specific course details including any recommended prepatory courses and Degree Requirements ...
... Please check the University Catalogue for specific course details including any recommended prepatory courses and Degree Requirements ...
Particles and interactions
... This is why the inner shell of any atom can contain at most two electrons. Electrons are fermions and so the Pauli exclusion principle applies to them. In the inner shell the one quantum number that can distinguish two electrons is the spin. Since the spin of the electron is ½, there are just two qu ...
... This is why the inner shell of any atom can contain at most two electrons. Electrons are fermions and so the Pauli exclusion principle applies to them. In the inner shell the one quantum number that can distinguish two electrons is the spin. Since the spin of the electron is ½, there are just two qu ...
Fulltext
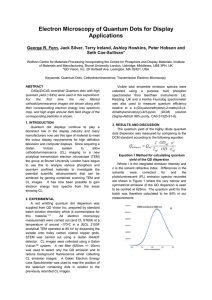
... the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show CL activity (e.g. region shown by the arrow in a and b and circled in c) but the smaller par ...
... the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show CL activity (e.g. region shown by the arrow in a and b and circled in c) but the smaller par ...
Electron Configuration Notes File
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
The Emergence of Quantum Mechanics
... the average behavior of tiny systems, rather than any given individual system, as if individual systems have no right to have any notion of reality attached to them. Nevertheless, it is also stressed that the theory can be extremely accurate; it is much more than a set of fuzzy assertions for object ...
... the average behavior of tiny systems, rather than any given individual system, as if individual systems have no right to have any notion of reality attached to them. Nevertheless, it is also stressed that the theory can be extremely accurate; it is much more than a set of fuzzy assertions for object ...
Rutherford Model 1911 - University of St Andrews
... other has spin “down” Therefore, 1st measurement determines (immediately) the result of the second, no matter how far apart the electrons are! But: perhaps this is the same as for a completely classical system - e.g. 2 boxes, one with something in it, the other empty. So make hypothesis: “each elect ...
... other has spin “down” Therefore, 1st measurement determines (immediately) the result of the second, no matter how far apart the electrons are! But: perhaps this is the same as for a completely classical system - e.g. 2 boxes, one with something in it, the other empty. So make hypothesis: “each elect ...
Slides - Professor Laura Ruetsche
... When we apply the quantization recipe to a classical field theory, we can obtain unitarily inequivalent representations of the CCRs encapsulating its quantization. Each purports to be the QFT that quantizes the classical field theory. Different quantizations can differ on such physically basic quest ...
... When we apply the quantization recipe to a classical field theory, we can obtain unitarily inequivalent representations of the CCRs encapsulating its quantization. Each purports to be the QFT that quantizes the classical field theory. Different quantizations can differ on such physically basic quest ...
Chapter 6
... Indicates the main energy level occupied by the electron Values are integers starting with 1 ...
... Indicates the main energy level occupied by the electron Values are integers starting with 1 ...
Chapter 12 Multiple Particle States
... has collapsed? Together with two other physicists, Podolsky and Rosen, Einstein argued that this behavior indicated that quantum theory had to be incomplete. In 1935, they published a paper describing what is now known as the “EPR Paradox” (Einstein et al., 1935). If quantum mechanics is indeed inco ...
... has collapsed? Together with two other physicists, Podolsky and Rosen, Einstein argued that this behavior indicated that quantum theory had to be incomplete. In 1935, they published a paper describing what is now known as the “EPR Paradox” (Einstein et al., 1935). If quantum mechanics is indeed inco ...