Terahertz-radiation-induced magnetic quantum ratchet effect in
... Si/SiO2. For optical experiments squared shaped samples (5 x 5 mm²) with ohmic contacts were ...
... Si/SiO2. For optical experiments squared shaped samples (5 x 5 mm²) with ohmic contacts were ...
Spin-Orbit Interaction - diss.fu
... cubic symmetry transformations at this point. Each of the three bands is also doubly degenerate in the spin, altogether a six-fold degeneracy at Γ. When spin-orbit interaction is included, the bands will split in two subbands with fourfold p3/2 and twofold p1/2 degeneracy (see Fig. 8.1(b)). The band ...
... cubic symmetry transformations at this point. Each of the three bands is also doubly degenerate in the spin, altogether a six-fold degeneracy at Γ. When spin-orbit interaction is included, the bands will split in two subbands with fourfold p3/2 and twofold p1/2 degeneracy (see Fig. 8.1(b)). The band ...
Introduction to Atomic Spectroscopy
... Splitting of the degenerate energy levels does take place for gaseous atoms in presence of a magnetic field. The complicated magnetic fields exerted by electrons in the matrix atoms and other species will affect the energy levels of analyte atoms. The simplest situation is one where an energy level ...
... Splitting of the degenerate energy levels does take place for gaseous atoms in presence of a magnetic field. The complicated magnetic fields exerted by electrons in the matrix atoms and other species will affect the energy levels of analyte atoms. The simplest situation is one where an energy level ...
Chapter_Superconductivity
... of the lattice through electrostatic coulomb force, some electron momentum get transferred. As a result, these ions set up elastic wave in the lattice due to distortion. If another electron happens to pass through this region then the interaction between two occurs which in its effect lowers the ene ...
... of the lattice through electrostatic coulomb force, some electron momentum get transferred. As a result, these ions set up elastic wave in the lattice due to distortion. If another electron happens to pass through this region then the interaction between two occurs which in its effect lowers the ene ...
Lecture 19: Building Atoms and Molecules
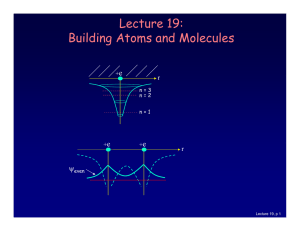
... which is described by an additional quantum number, mp, and therefore also a magnetic moment. However, it is several orders of magnitude smaller than that of the electron. ...
... which is described by an additional quantum number, mp, and therefore also a magnetic moment. However, it is several orders of magnitude smaller than that of the electron. ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... 6. In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? A. Principal quantum number (n) which describes the energy level/distance from the nucleus B. Heisenberg number (H) which describes t ...
... 6. In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? A. Principal quantum number (n) which describes the energy level/distance from the nucleus B. Heisenberg number (H) which describes t ...
Lecture 19: Building Atoms and Molecules
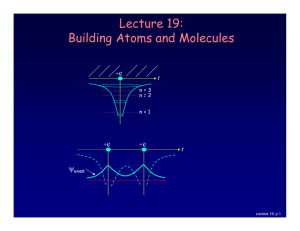
... which is described by an additional quantum number, mp, and therefore also a magnetic moment. However, it is several orders of magnitude smaller than that of the electron. ...
... which is described by an additional quantum number, mp, and therefore also a magnetic moment. However, it is several orders of magnitude smaller than that of the electron. ...
Lewis
... Molecular structure and bonding To understand the formation and structure of molecular compounds, first one has to learn, recognize, use, count, take into account: • the periodic table with groups and periods, • the number of electrons and valence electrons (i.e. count electrons), (2 (K), 8 (L) = 2 ...
... Molecular structure and bonding To understand the formation and structure of molecular compounds, first one has to learn, recognize, use, count, take into account: • the periodic table with groups and periods, • the number of electrons and valence electrons (i.e. count electrons), (2 (K), 8 (L) = 2 ...
Outline_CH16_Klein
... 1) Nuclear Spin and Magnetic Resonant Frequency A) Spin Angular Momentum and Magnetic Moments o Spin quantum number I = ½ nuclei o Odd atomic number or odd mass number I≠0 o Spin ½ nuclei have 2 spin degenerate spin states and o When nucleus is not in the presence of an external magnetic field, ...
... 1) Nuclear Spin and Magnetic Resonant Frequency A) Spin Angular Momentum and Magnetic Moments o Spin quantum number I = ½ nuclei o Odd atomic number or odd mass number I≠0 o Spin ½ nuclei have 2 spin degenerate spin states and o When nucleus is not in the presence of an external magnetic field, ...
IV. MICROWAVE SPECTROSCOPY R. D. Mattuck
... A resonant cavity was added to the molecular-beam spectroscope in order to allow more efficient matching of the microwave power into the sodium chloride beam. The inner dimensions of this cavity (see Fig. IV-1) are: ...
... A resonant cavity was added to the molecular-beam spectroscope in order to allow more efficient matching of the microwave power into the sodium chloride beam. The inner dimensions of this cavity (see Fig. IV-1) are: ...
wall_summer_2011_poster
... 1. Light is generated in a gas discharge tube which is located between the poles of the magnet. 2. The light then passes through the slit. 3. After passing through the slit the light is reflected by the focusing mirror. The slit is located at the focal length of the focusing mirror, and as a result ...
... 1. Light is generated in a gas discharge tube which is located between the poles of the magnet. 2. The light then passes through the slit. 3. After passing through the slit the light is reflected by the focusing mirror. The slit is located at the focal length of the focusing mirror, and as a result ...
powerpoint
... two moments, orbital energy can be slightly altered. We use the so-called Na D line as a paradigm. We use the first-order perturbation theory to describe the shifts in orbital energies. The spin-orbit interaction is a relativistic effect and its derivation is beyond the scope of this course. We trea ...
... two moments, orbital energy can be slightly altered. We use the so-called Na D line as a paradigm. We use the first-order perturbation theory to describe the shifts in orbital energies. The spin-orbit interaction is a relativistic effect and its derivation is beyond the scope of this course. We trea ...
Word - ASDL Community
... transition and to the energy of a nuclear spin flip? What are the consequences of your answers to these questions? 4. If thermal energy has sufficient energy to excite nuclear spin flips, why are there still more in the ground than excited state? 5. Can you think of two processes by which a specific ...
... transition and to the energy of a nuclear spin flip? What are the consequences of your answers to these questions? 4. If thermal energy has sufficient energy to excite nuclear spin flips, why are there still more in the ground than excited state? 5. Can you think of two processes by which a specific ...
Nitrogen-vacancy center

The nitrogen-vacancy center (N-V center) is one of numerous point defects in diamond. Its most explored and useful property is photoluminescence, which can be easily detected from an individual N-V center, especially those in the negative charge state (N-V−). Electron spins at N-V centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field, electric field, microwave radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin-orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual N-V center can be viewed as a basic unit of a quantum computer, and it has potential applications in novel, more efficient fields of electronics and computational science including quantum cryptography and spintronics.