Don`t Change - Dr. Lodge McCammon
... Helps photosynthesis keep things growing How are photosynthesis and electromagnetic energy connected? Electromagnetic energy (sunlight) is required for photosynthesis to occur. Write down the equation for photosynthesis __________________________________________ Carbon dioxide plus water oxygen an ...
... Helps photosynthesis keep things growing How are photosynthesis and electromagnetic energy connected? Electromagnetic energy (sunlight) is required for photosynthesis to occur. Write down the equation for photosynthesis __________________________________________ Carbon dioxide plus water oxygen an ...
Reduction and Oxidation
... bond. It can be considered as the charge that an atom would have if the more electronegative atom in a bond obtains the two electrons of the bond completely. When an element is ascribed a particular oxidation number, it is called the element is in a specific oxidation sta ...
... bond. It can be considered as the charge that an atom would have if the more electronegative atom in a bond obtains the two electrons of the bond completely. When an element is ascribed a particular oxidation number, it is called the element is in a specific oxidation sta ...
Environmental Effects on Atomic Energy Levels.
... forces. These forces are additive in the approximation of second-order perturbation theory, which is as far as we have carried the analysis. But application of third-order perturbation theory does not lead to additive energies. It seems probable, however, that this error is not large in most cases o ...
... forces. These forces are additive in the approximation of second-order perturbation theory, which is as far as we have carried the analysis. But application of third-order perturbation theory does not lead to additive energies. It seems probable, however, that this error is not large in most cases o ...
"Compression" of the electron shell of a neutral atom by a crystal
... y resonance method (Miissbauer spectroscopy). It was shown that the impurity tin atoms in loose Ga2Te3type structures were in nonionized atomic states and the compression of their electron shells in the crystal lattice led to a substantial increase in the charge density around SnlL9nuclei. A simple ...
... y resonance method (Miissbauer spectroscopy). It was shown that the impurity tin atoms in loose Ga2Te3type structures were in nonionized atomic states and the compression of their electron shells in the crystal lattice led to a substantial increase in the charge density around SnlL9nuclei. A simple ...
1H-NMR and 13C-NMR Spectra - Royal Society of Chemistry
... bending vibration of the C–F bond at the axial ligand. The In–C stretching mode can not be unambiguously assigned due to coupling with C– C modes and vibrations of the phthalocyanine macrocycle. Pure dib shows that the NC stretching mode appears at 2132 cm -1, whereas the NC absorption in 4 and 5 a ...
... bending vibration of the C–F bond at the axial ligand. The In–C stretching mode can not be unambiguously assigned due to coupling with C– C modes and vibrations of the phthalocyanine macrocycle. Pure dib shows that the NC stretching mode appears at 2132 cm -1, whereas the NC absorption in 4 and 5 a ...
Show all work – Homework 5 –
... 1. Transition metal oxides containing a metal ion with multiple oxidation states may be non-stoichiometric. The non-stoichiometric compound may accommodate this by a variety of ways, including cation vacancies, oxide ion (anion) vacancies, interstitial cations, or interstitial anions. Soft chemistry ...
... 1. Transition metal oxides containing a metal ion with multiple oxidation states may be non-stoichiometric. The non-stoichiometric compound may accommodate this by a variety of ways, including cation vacancies, oxide ion (anion) vacancies, interstitial cations, or interstitial anions. Soft chemistry ...
Plasmons, polaritons What are plasmons and what are
... because their plasmon frequency is in the ultraviolet. Some metals, such as copper and gold, have electronic interband transitions in the visible range, whereby specific energies (colors) are absorbed. Thus, those metals have a distinct color. Polaritons: Polaritons are bosonic (quasi‐)particles ...
... because their plasmon frequency is in the ultraviolet. Some metals, such as copper and gold, have electronic interband transitions in the visible range, whereby specific energies (colors) are absorbed. Thus, those metals have a distinct color. Polaritons: Polaritons are bosonic (quasi‐)particles ...
Laboratory 15: Spectroscopy d m = × 167 10 .
... angle is observed at which the first order (n = 1) image of a certain color occurs and the grating spacing is known, the wave-length of a that color of light can be determined from equation 1. We will examine two different light sources, mercury and hydrogen. In these sources a high voltage sent thr ...
... angle is observed at which the first order (n = 1) image of a certain color occurs and the grating spacing is known, the wave-length of a that color of light can be determined from equation 1. We will examine two different light sources, mercury and hydrogen. In these sources a high voltage sent thr ...
論文の構成 - 秋山研究室
... At intermidiate ne, the peak of trion stays at the same energy with ne while the absorption peak at high ne blue-shifts rapidly from the higher energy side of the trion peak. In other words, the absorption peak at high ne does not originate from the trion peak. This is interesting because, in 2D ele ...
... At intermidiate ne, the peak of trion stays at the same energy with ne while the absorption peak at high ne blue-shifts rapidly from the higher energy side of the trion peak. In other words, the absorption peak at high ne does not originate from the trion peak. This is interesting because, in 2D ele ...
excited states
... All terms except kf must be minimized to obtain a large fluorescent signal. Chemical structures that minimize one or more of the these rate constants increase the quantum yield. Structural rigidity: Decreases the chances of vibrational and rotational de-excitation (which we have called IC (internal ...
... All terms except kf must be minimized to obtain a large fluorescent signal. Chemical structures that minimize one or more of the these rate constants increase the quantum yield. Structural rigidity: Decreases the chances of vibrational and rotational de-excitation (which we have called IC (internal ...
Microsoft Word Format - University of Toronto Physics
... the total electronic spin S = 1/2, there are, in this state, two possible values of the total electronic angular momentum, J = 3/2 and J = 1/2. Due to the spin-orbit interaction, these two states of different total angular momentum differ in energy relative to the ground state which gives rise to th ...
... the total electronic spin S = 1/2, there are, in this state, two possible values of the total electronic angular momentum, J = 3/2 and J = 1/2. Due to the spin-orbit interaction, these two states of different total angular momentum differ in energy relative to the ground state which gives rise to th ...
Optical spectroscopy techniques
... Pump and Probe beam configuration: Doppler-‐broadened shape can be eliminated ...
... Pump and Probe beam configuration: Doppler-‐broadened shape can be eliminated ...
VI Zagrebaev and VV Samarin
... CCFULL code is displayed in Fig. 1. As can be seen, the two codes in question lead to nearly identical values for the same interaction parameters. In the case of fusion of spherical nuclei with excitation of their vibrational states both codes also give almost identical results. In the fusion of ver ...
... CCFULL code is displayed in Fig. 1. As can be seen, the two codes in question lead to nearly identical values for the same interaction parameters. In the case of fusion of spherical nuclei with excitation of their vibrational states both codes also give almost identical results. In the fusion of ver ...
Instruction Manual PH511: Physics Laboratory-III DEPARTMENT OF PHYSICS
... Geiger tube is one of the oldest and widely used nuclear radiation detectors. It consists of a metallic tube with a thin wire mounted along its axis. The wire is insulated from the tube using a ceramic feed-through (Fig. 3.1). The central wire (anode) is kept at a positive potential of a few hundred ...
... Geiger tube is one of the oldest and widely used nuclear radiation detectors. It consists of a metallic tube with a thin wire mounted along its axis. The wire is insulated from the tube using a ceramic feed-through (Fig. 3.1). The central wire (anode) is kept at a positive potential of a few hundred ...
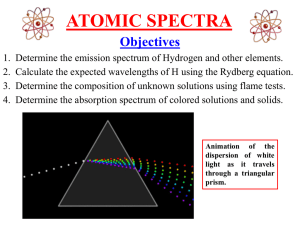
Atomic_spectra
... 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. ...
... 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. ...
Structure and optical properties of reconstructed Si and Ge surfaces
... as the forces on the bulk atoms are not similar to those on surface atoms. This is called surface reconstruction. When these systems are energetically relaxed, sometimes they form special ordered structures such as one dimensional atomic chains or rows of dimers etc. due to surface reconstruction. S ...
... as the forces on the bulk atoms are not similar to those on surface atoms. This is called surface reconstruction. When these systems are energetically relaxed, sometimes they form special ordered structures such as one dimensional atomic chains or rows of dimers etc. due to surface reconstruction. S ...
AC Circuits
... In this part of the experiment, you need to measure the absorption spectrum of the Rose Bengal solution at different concentrations (Rose Bengal is an organic dye). Prepare a master aqueous solution of molar concentration ~1.25x10-4 M (moles/l) in a large storage vial. Use the plastic pipettes to tr ...
... In this part of the experiment, you need to measure the absorption spectrum of the Rose Bengal solution at different concentrations (Rose Bengal is an organic dye). Prepare a master aqueous solution of molar concentration ~1.25x10-4 M (moles/l) in a large storage vial. Use the plastic pipettes to tr ...
NicholasBarbutoPoster - Physics
... usually referred to as the band gap. This stems from the periodic nature of the atoms or molecules in a crystal lattice. In photonic crystals, the same type of periodic structure exists, only in place of electron conduction properties, the dielectric properties, or photon conduction properties are p ...
... usually referred to as the band gap. This stems from the periodic nature of the atoms or molecules in a crystal lattice. In photonic crystals, the same type of periodic structure exists, only in place of electron conduction properties, the dielectric properties, or photon conduction properties are p ...
Columbus Conference
... State of an ion or molecule where an excited electron has a high principal quantum number Hydrogenic in nature, with a binding energy given as: ...
... State of an ion or molecule where an excited electron has a high principal quantum number Hydrogenic in nature, with a binding energy given as: ...
Snímek 1
... suppressed by competitive positron beta decay. Emission of couple of protons – made by coupling (maybe also in form of 2He) - year 2000 at Oak Ridge laboratory for nucleus 18Ne Delayed proton emission – emission of protons following after proton decay → nuclei with large excess of protons → created ...
... suppressed by competitive positron beta decay. Emission of couple of protons – made by coupling (maybe also in form of 2He) - year 2000 at Oak Ridge laboratory for nucleus 18Ne Delayed proton emission – emission of protons following after proton decay → nuclei with large excess of protons → created ...
6. Molecular vibrations
... excitation energies the parabolic approximation is poor (the true potential is less confining) and is totally wrong near the dissociation ...
... excitation energies the parabolic approximation is poor (the true potential is less confining) and is totally wrong near the dissociation ...
Absorption 1
... H2O and O3), there are three normal modes – also known as fundamentals. For linear molecules such as CO2 and NO2, there are four (!) fundamentals, but two orthogonal bending modes are degenerate and so only three fundamentals exist. The term “degenerate” should not be taken personally – it just mean ...
... H2O and O3), there are three normal modes – also known as fundamentals. For linear molecules such as CO2 and NO2, there are four (!) fundamentals, but two orthogonal bending modes are degenerate and so only three fundamentals exist. The term “degenerate” should not be taken personally – it just mean ...
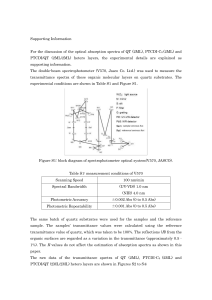
Supporting Information For the discussion of the optical absorption
... In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT HOMO level drops to a lower level or the optical band-gap expands after exposure to air. In an ...
... In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT HOMO level drops to a lower level or the optical band-gap expands after exposure to air. In an ...
Cold Fusion By Plasma Electrolysis of Water
... truth [6] ? There is no exact answer, but it is known that the Japanese scientists registered only neutron radiation with intensity of 50,000 neutrons per second, and they failed to register roentgen radiation [4]. If in this process the roentgen photons were created, they would not exceed heat effi ...
... truth [6] ? There is no exact answer, but it is known that the Japanese scientists registered only neutron radiation with intensity of 50,000 neutrons per second, and they failed to register roentgen radiation [4]. If in this process the roentgen photons were created, they would not exceed heat effi ...
Mössbauer spectroscopy

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1957, consists in the recoil-free, resonant absorption and emission of gamma rays in solids.Like NMR spectroscopy, Mössbauer spectroscopy probes tiny changes in the energy levels of an atomic nucleus in response to its environment. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting, also known as the Zeeman effect. Due to the high energy and extremely narrow line widths of gamma rays, Mössbauer spectroscopy is a very sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting change in just a few parts per 1011.