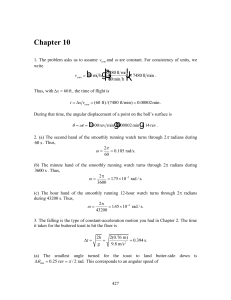
Noncovalent interactions of molecules with single walled carbon
... energy.25,26 However, addition of an atom to the interior of the sidewall would add strain energy to the nanotube. Theoretical modelling has shown that the interior of a SWNT is more inert than the exterior of a nanotube to the addition of atomic nitrogen,27 atomic carbon,28 atomic fluorine,26 atomi ...
... energy.25,26 However, addition of an atom to the interior of the sidewall would add strain energy to the nanotube. Theoretical modelling has shown that the interior of a SWNT is more inert than the exterior of a nanotube to the addition of atomic nitrogen,27 atomic carbon,28 atomic fluorine,26 atomi ...
Density Functional Study of Molecular Orbitals of Ferrocene and
... each carbon atom of (C5H5)-are involved in the formation of σ bond between C-C and C-H. The orbitals involved in σ bond hence shall remain out of discussion. The 2pz orbitals of ten carbons and nine orbitals of iron or cobalt i.e., in total nineteen orbitals are relevant to our discussion in respect ...
... each carbon atom of (C5H5)-are involved in the formation of σ bond between C-C and C-H. The orbitals involved in σ bond hence shall remain out of discussion. The 2pz orbitals of ten carbons and nine orbitals of iron or cobalt i.e., in total nineteen orbitals are relevant to our discussion in respect ...
Density Functional Study of Molecular Orbitals of
... of reactivity, shows cobaltocene has lower chemical reactivity comparing to nickelocene molecule. Absolute hardness and softness are important properties to measure the molecular stability and reactivity. It is apparent that the chemical hardness fundamentally signifies the resistance towards the de ...
... of reactivity, shows cobaltocene has lower chemical reactivity comparing to nickelocene molecule. Absolute hardness and softness are important properties to measure the molecular stability and reactivity. It is apparent that the chemical hardness fundamentally signifies the resistance towards the de ...
Raman Spectroscopy on Semiconductor Nanowires
... Studies comparing Raman scattering experiments of bulk and nanostructured materials have been reported in literature for several different kind of systems. It is usually observed that the transversal optical (TO) and the longitudinal optical (LO) modes have a position in energy close to that observe ...
... Studies comparing Raman scattering experiments of bulk and nanostructured materials have been reported in literature for several different kind of systems. It is usually observed that the transversal optical (TO) and the longitudinal optical (LO) modes have a position in energy close to that observe ...
Quasi Classical Trajectory Binning: A Systematic
... Here nvf ,jf is the e↵ective number of classical trajectories that are said to have made the transition from the state (vi , ji ) to the state (vf , jf ), and N is the total number of trajectories. As we will discuss later, calculating nvf ,jf from the distribution of final classical states is one o ...
... Here nvf ,jf is the e↵ective number of classical trajectories that are said to have made the transition from the state (vi , ji ) to the state (vf , jf ), and N is the total number of trajectories. As we will discuss later, calculating nvf ,jf from the distribution of final classical states is one o ...
Perspectives on the Physical Chemistry of
... The properties of crystalline solids are ordinarily catalogued without reference to their size. It is only in the regime below 10 nm where this variable comes into play. In the past decade, tailoring of materials characteristics by size control has been demonstrated in many inorganic solids belongin ...
... The properties of crystalline solids are ordinarily catalogued without reference to their size. It is only in the regime below 10 nm where this variable comes into play. In the past decade, tailoring of materials characteristics by size control has been demonstrated in many inorganic solids belongin ...
24_(582-588)(완료)E14120 ed-Jin-Kyu Yang.hwp
... how the dipole emitter is close to the LSP mode, spatially and spectrally. We also calculated the absorption and resonance behaviors at Ag/SiO2 CS NPs in both the TM-polarized dipole and the TE-polarized dipole. Figure 3(a) shows the absorption spectra when the radius of Ag is 15 nm. The absorption ...
... how the dipole emitter is close to the LSP mode, spatially and spectrally. We also calculated the absorption and resonance behaviors at Ag/SiO2 CS NPs in both the TM-polarized dipole and the TE-polarized dipole. Figure 3(a) shows the absorption spectra when the radius of Ag is 15 nm. The absorption ...
Electronic spectra and hyperpolarizabilities of structurally similar
... large electron density on the indole group (phenyl group containing the N centre in DN2) relative to the phenyl group at the other side of the molecule. On the other hand, the relative electron density on the phenyl group at the other side is seen to increase in the LUMO of the dyes. This suggests t ...
... large electron density on the indole group (phenyl group containing the N centre in DN2) relative to the phenyl group at the other side of the molecule. On the other hand, the relative electron density on the phenyl group at the other side is seen to increase in the LUMO of the dyes. This suggests t ...
Torque Spectroscopy for the Study of Rotary Motion in Biological
... Figure 2. Overview of single-molecule twist and torque measurement approaches. (a−c) Viscous drag-based methods. (a) An E. coli cell body (green) is attached by its flagellum (black) to a glass slide (blue). The rotation of the cell body is used to read out the operation of the bacterial flagellar mot ...
... Figure 2. Overview of single-molecule twist and torque measurement approaches. (a−c) Viscous drag-based methods. (a) An E. coli cell body (green) is attached by its flagellum (black) to a glass slide (blue). The rotation of the cell body is used to read out the operation of the bacterial flagellar mot ...
(NH3)n and NH2 - Sanov Group
... VDE between a cluster and the bare anion is an approximate measure of the solvent stabilization of the anion, while drastic spectral changes along a solvation series may indicate chemistry or physics beyond those of electrostatic ion-solvent interactions. The effects of solvation on the correspondin ...
... VDE between a cluster and the bare anion is an approximate measure of the solvent stabilization of the anion, while drastic spectral changes along a solvation series may indicate chemistry or physics beyond those of electrostatic ion-solvent interactions. The effects of solvation on the correspondin ...
Rotational spectroscopy

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously.For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and symmetric top; analytical expressions can be derived for the rotational energy terms of these molecules. Analytical expressions can be derived for the fourth category, asymmetric top, for rotational levels up to J=3, but higher energy levels need to be determined using numerical methods. The rotational energies are derived theoretically by considering the molecules to be rigid rotors and then applying extra terms to account for centrifugal distortion, fine structure, hyperfine structure and Coriolis coupling. Fitting the spectra to the theoretical expressions gives numerical values of the angular moments of inertia from which very precise values of molecular bond lengths and angles can be derived in favorable cases. In the presence of an electrostatic field there is Stark splitting which allows molecular electric dipole moments to be determined.An important application of rotational spectroscopy is in exploration of the chemical composition of the interstellar medium using radio telescopes.